New cheap CRS-blockers at the horizon
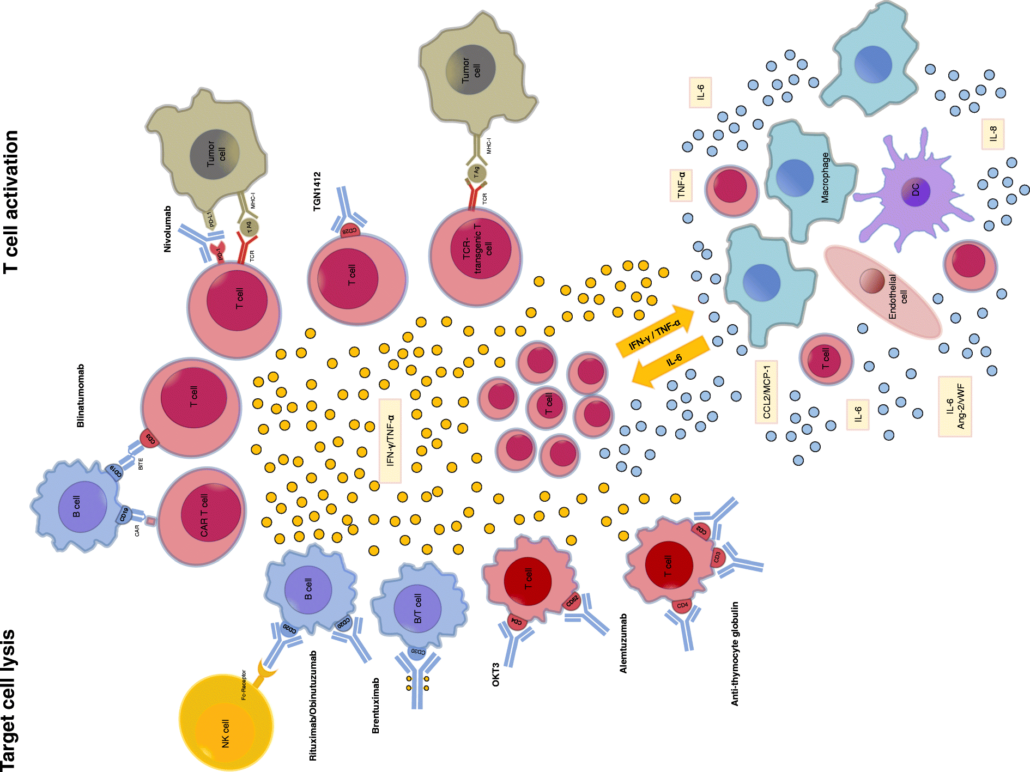
Cancer immunotherapies are often associated with severe cytokine release syndrome (CRS) that can be managed with IL-6R-blockers. US oncologists now report that an FDA-approved hypertension drug did the same job as Roche's anti-IL-6R blocker tocolizumab.
In mice, the team headed by Bert Vogelstein and Shibin Zhou from Johns-Hopkins University in Baltimore demonstrated that severe CRS was linked to an increase in catecholamines levels, such as adrenaline. Catecholamine levels and CRS symptoms decreased, however, when the oncologists administered mice with Burkitt lymphoma or B-ALL, which had been treated with CD-19 CAR T cells, anti-CD3-mAbs or T-cell activating bispecific antibodies, with the FDA-approved catecholamine synthesis blocker metyrosine (Demser). What’s more, the hypertensive drug did not affect the efficacy of the cancer immune therapies.
Furthermore Demser prevented adrenaline-induced CRS in mice. 100 capsules with 250mg of the anti-hypertensive drug costs roughly US$35, while a 800mg injection of tocolizumab (Roche/Genentech), which is FDA-approved to manage therapy-induced CRS, costs about US$465.
Deadly CRS induced by oncolytic Clostridium-bacteria could be also prevented in 80% of mice with colon cancer without reducing efficacy using a drug, developed by Palantin Technologies that is due to enter Phase IIa clinical testing. The synthetic atrial natriuretic peptide precursor A mimic called PL-3994 reduced CRS-related high catecholamine levels and decreased necrosis, cytokine production and immune cell infiltration.
"Our study identifies catecholamines as an essential component of the cytokine release that can be modulated by specific blockers without impairing the therapeutic response," conclude the researchers who now suggest to evaluate metyrosine clinically to prevent CRS. The alternative would be highly welcome to treat patients with CRS who are tocolizumab-refractive.



 Boehringer Ingelheim
Boehringer Ingelheim Olga Yastremska, New Africa, freepik
Olga Yastremska, New Africa, freepik