Novartis acquires inflammasome blocker company IFM Tre
Novartis has aquired all outstanding capital stock and paid US$310m upfront to Boston-based IFM Tre Inc., which commercialises three inflammasome blockers designed to treat chronic inflammatory diseases.
IFM Therapeutics LLC spin-out IFM Tre raised $31m in July following the acquisition of its mother company and its NRLP3-blocking cancer assets by BMS. The acquisition gives Novartis the global commercialisation rights of three small molecule blockers of the NLRP3 inflammasome, the clinical programme IFM-2427 and two programmes in preclinical development stage.
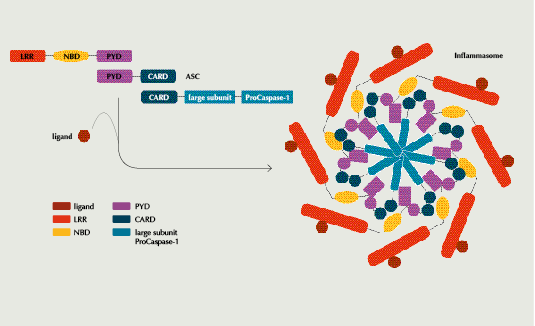
The NRLP3 inflammasome is an intracellularly formed danger-sensing multiprotein complex of myeloid cells of the innate immune system that triggers IL-1?-mediated inflammatory responses. Mutations in the NLR genes, which encode 23 inflammasome proteins that detect different cellular threats using pathogen-associated molecular patterns (PAMP) or damage-associated molecular patterns (DAMP), have been shown to lead to chronic inflammation. Blocking inflammasome activity thus promises to reduce cytokine overproduction and stop inflammatory signaling that could initiate fibrosis, NASH, atherosclerosis or other common chronic diseases.
Under the agreement, Novartis will acquire all of the outstanding capital stock of IFM Tre, Furthermore, IFM Tre will receive $310m upfront and is eglible to receive further $1.3bn in potential milestone payments upon commercialisation of its candidates. IFM Tre expects read-out from an expanded Phase I clinical trial enroling 90 volunteers for IFM-2427 safety testing at the end of the year. IFM-2427 is designed to treat chronic inflammatory diseases including gout, atherosclerosis and non-alcoholic steatohepatitis (NASH), which might complement some Novartis’ candidate drugs. IFM Tre’s preclinical compounds target IBD and neurodegenerative diseases.
Last November, Roche’s US affiliate Genentech acquired the NLPR3 inhibitor developer Jecure Therapeutics, which has preclinical NLRP3 blockers to treat NASH and liver fibrosis in its pipeline. Other inflammasome inhibitor developers have announced to start clinical testing in 2019.


 Bayer AG
Bayer AG
 Picture from Ferdinand Stöhr on Unsplash
Picture from Ferdinand Stöhr on Unsplash