AM-Pharma raises €116m for Phase III
Dutch biopharma company AM-Pharma B.V. has secured €116m to bring its acute kidney injury treatment into Phase III.
In a Phase II trial, the recombinant human Alkaline Phosphatase (recAP) showed promise, even though it missed its primary endpoint. After a buyout deal with Pfizer fell through, AM-Pharma is now moving into Phase III on its own. The Bunnik-based company will use the funds to carry out a multi-national pivotal Phase III trial of recAP in 1,400 patients with sepsis-associated acute kidney injury. The financing was co-led new investors LSP and Andera Partners, with existing investors Forbion, Ysios Capital, Kurma Partners, ID Invest Partners, BB Pureos Bioventures and Gilde Healthcare also participating.
Acute kidney injury – for which sepsis is the major cause – currently has no approved pharmacological treatments. recAP has the potential to be a first-in-class for this indication. In 2016, AM-Pharma was awarded Fast Track designation by the US Food and Drug Administration. recAP has the potential to become the first pharmacological treatment and a blockbuster first-line therapy for critically ill patients with SA-AKI, commented Martijn Kleijwegt, Managing Partner at LSP. With its promising Phase II recAP data published last year, this is an exciting time to support the Company as it is poised and ready to start the Phase III clinical study.
Erik van den Berg, AM-Pharma’s CEO added: Raising this amount of capital from highly experienced life sciences venture capital firms LSP and Andera Partners, with continued support from our existing investors, highlights the urgent medical need in AKI and the potential of recAP as a life-saving treatment. We look forward to working with our expanded investor syndicate and to initiating the Phase III trial.
To date, AM-Pharma has raised over €195m in equity and debt.


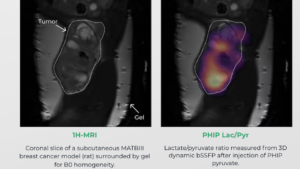 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics