
Pharming licenses orphan drug from Novartis
Pharming NV has paid €17.9m upfront to Novartis AG for an exclusive license to CDZ173, a late stage drug for the treatment of APDS
Novartis AG’s small molecule phosphoinositide 3-kinase delta (PI3K?) blocker CDZ173, which is in a registration-enabling study, is expected to reach the market in 2022 lastest. Activated Phosphoinositide 3-kinase Delta Syndrome (APDS) is an ultra-rare, debilitating primary immune deficiency which makes carriers of the underlying defect in the PIK3CD gene unable to fight of infections. Currently there is no approved treatment available for PIK3CD overactivity, which occurrs approximately 1-2 per million.
Importantly, there is a commercially available genetic test that can identify the patients who will benefit from CDZ173 making this program personalized medicine. Novartis has completed all the preclinical and clinical work to date and will continue to run the ongoing registration-enabling trial and the ongoing open label extension study. Pharming will work alongside Novartis to complete enrollment of the ongoing trial. Upon approval, Pharming will commercialize CDZ173 through its existing commercial infrastructure in the US and Europe and look for ways to make the drug available in other markets worldwide.


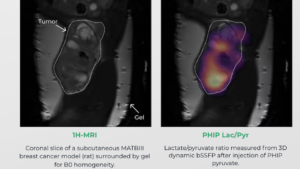 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics