DPP3 triggers myocardial depression
Results presented at European Society of Cardiology Congress 2019 demonstrate a new disease mechanism predicting acute organ dysfunction in heart failure patients.
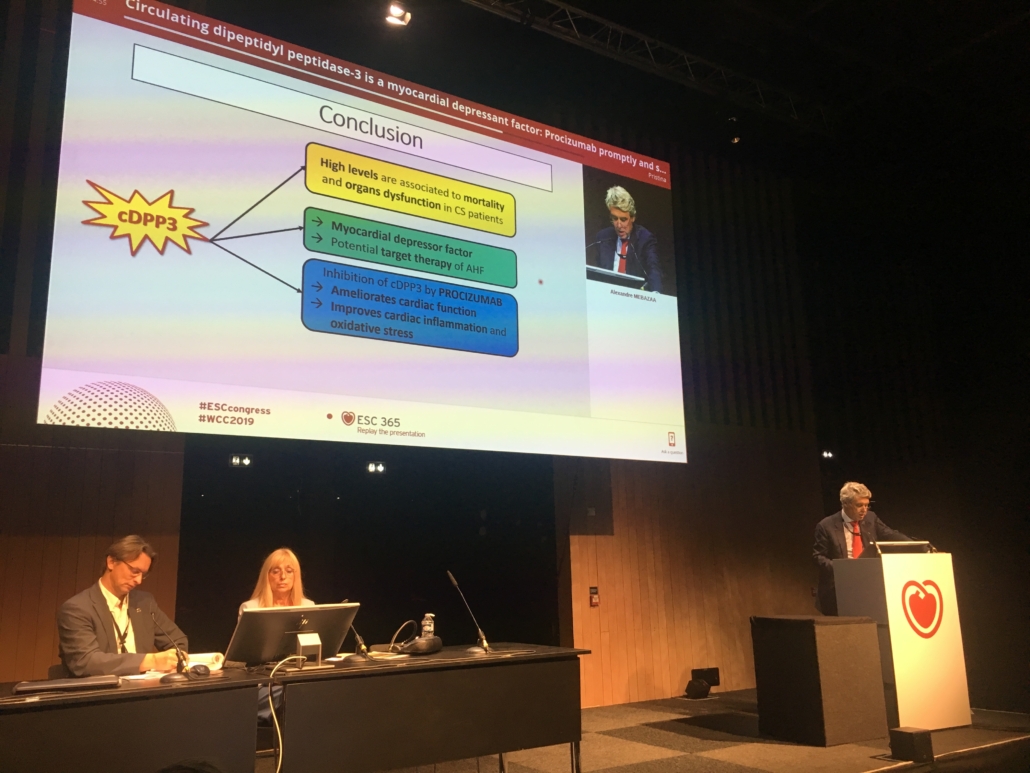
Results of two independent studies published fast-track and presented on Saturday at ESC Congress 2019 (Paris) by Prof. Alexandre Mebazza (Hôpital Lariboisière, Paris) and the companies Sphingotec GmbH and 4TEEN4 Pharmaceuticals demonstrate that dipeptidyl peptidase 3 (DPP 3) is at the core of a novel disease mechanism for organ failure in cardiogenic shock. DPP3 degrades the cardiovascular mediators angiotensin II and enkephalin, which are central to heart and kidney function. Degradation of angiotensin II and enkephalin leads to acute, short-term organ dysfunction and death.
According to Mebazza, high blood concentrations of DPP3 at admission to intensive care units (ICUs) were associated with organ dysfunction and high mortality risk in patients with cardiogenic shock, the most severe form of cardiac depression. High DPP3 blood concentrations also correlated with the development of refractory shock. Mebazaa and colleagues hypothesize that intracellular DPP3 is released upon massive necrotic cell death in critically ill patients. In preclinical models of acute heart failure, injections of DPP3 decreased cardiac output, as well as kidney hemodynamics. Administration of the anti-DPP3 antibody procizumab. developed by 4TEEN4, immediately normalised angiotensin II and enkephalin levels and reversed acute short-term organ dysfunction due to decreased cardiac output and impaired kidney hemodynamics.
Mebazza said: DPP3 represents a unique biomarker at the core of a novel disease mechanism with high potential utility in diagnosing organ dysfunction and predicting outcome in cardiogenic shock as well as in a
range of other acute care indications." According to Mebazza, "inhibition of DPP3 in cardiogenic shock patients might restore myocardial function and save lives".
At the ESC, Sphingotec launched IB10 sphingotest® DPP3, the first CE-IVD-marked point-of-care biomarker test able to quantify DPP3 blood-plasma levels. The test is designed and validated for use with sphingotec’s fully automated Nexus IB10 whole blood point-of-care platform for rapid testing in laboratories, emergency
departments and intensive care units together with a menu of standard critical care tests also available
on the Nexus IB10 instrument. Though Spingotec has already DPP3 data from about 20.000 patients in several indications caused by angiotensin II and enkephalin pathway disruptions, which will be published step-by-step, the company invited the critical care community at ESC Congress to further validate where DPP3 could provide clinical utility.
4TEEN4 is currently setting up a GMP process for Procizumab manufacturing and is expected to enter
regulatory non-clinical safety studies next year. The company has licensed the global rights to develop
DPP3-based diagnostics for near-patient and central laboratory testing to SphingoTec GmbH
(sphingotec; Hennigsdorf, Germany). 4TEEN4 and sphingotec, together with Nexus Dx Inc. and
Adrenomed AG, are part of the Medicine4Future Initiative established by serial entrepreneur Dr.
Andreas Bergmann.


 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics