Fighting sepsis mortality
Critical care experts have presented data supporting the thesis that three specific pathways are the cause for about 90% of sepsis mortality.
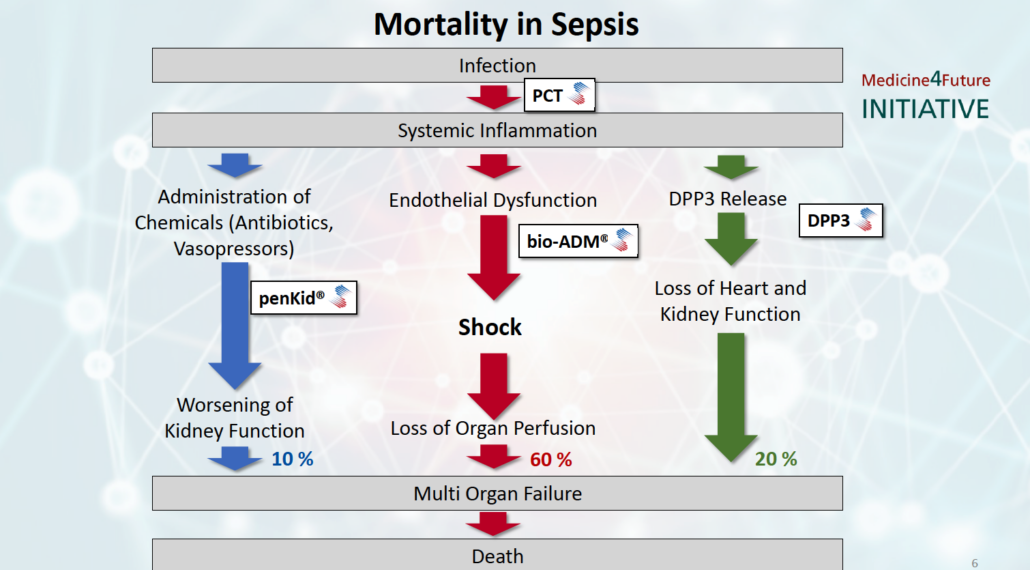
For decades, the causes of high sepsis mortality have remained obscure. In mid-December, critical care experts from clinical practice and industry presented data demonstrating that three mortality mechanisms may cover the majority of sepsis deaths (90%). At the scientific symposium Mortality in Sepsis in Berlin, hosted by diagnostics company SphingoTec GmbH (Hennigsdorf),together with Adrenomed AG and 4TEEN4 Pharmaceuticals, Professor Konrad Reinhart, Founding President of the Global Sepsis Alliance, stressed that sepsis is an medical emergency that is still underserved because there are no approved causal treatments available and diagnostic results are obtained too late. Novel predictive biomarkers, some of which can be targeted with therapeutic antibodies, however, may herald in a paradigm change towards personalized sepsis treatment in the future, concluded the 30 key opinion leaders and industry experts at the symposium.
Every 3.5 seconds, one in the 30.7 million patients annually diagnosed with sepsis, dies. Speaking at the scientific symposium Mortality in Sepsis, Reinhart stressed that the true burden of the systemic inflammation that results in organ dysfunction and its estimated six million deaths per year is largely underestimated as the ICD-9 sepsis coding represented only a fraction of cases (i.e. US 30%, Sweden 15%). He pointed out that measures such as (pneumonia) vaccination, early warning scores and sepsis awareness campaigns pushed by the World Health Organization (WHO) since 2017 have already decreased mortality rates in early adopting countries such as Australia, the US and the UK. However, what has not changed for decades is the lack of early biomarkers that allow the identification of risk patients susceptible to septic shock who require aggressive medical intervention right from the start when admitted to emergency departments (EDs) and intensive care units (ICUs).
A growing body of evidence presented at the symposium suggest that novel point-of-care diagnostic (POC) tests developed within the Medicines4Future initiative allow timely patient stratification opening up the avenue to a more personalized therapeutic intervention that might significantly cut sepsis mortality.
In Berlin, Dr Andreas Bergmann, founder of SphingoTec GmbH, Adrenomed AG and 4TEEN4 Pharmaceuticals GmbH, presented the Medicines4Future Initiative he started ten years ago. Within Medicines4Future we seek to explore the causative pathways of mortality in sepsis systematically, he explained, and based on prospective observation studies and subsequent validation studies we identified certain mechanisms of mortality. Ten years after Bergmann and co-founders sold diagnostics company B.R.A.H.M.S. AG, where the serial founder developed the standard sepsis marker procalcitonin (PCT), to Thermo Fisher Scientific for €330 million, three distinguishable mortality pathways have materialized within the Medicines4Future initiative. While SphingoTec is launching CE-marked in vitro diagnostics (IVD) tests running on the company’s proprietary point-of-care (POC) Nexus IB10 platform along with standard tests for critical care such as Troponin, NT-proBNP etc. to provide the critical care community with tests that identify the disrupted pathway, Adrenomed and 4TEEN4 provide therapeutic antibodies that may correct the underlying dysfunction.
Biomarkers for decision support in critical care
One cause of mortality identified within the Medicine4Future Initiative is cell necrosis-induced heart depression and kidney dysfunction caused by release of the intracellular enzyme dipeptidyl peptidase 3 (DPP3) into the bloodstream. There, DPP3 cleaves two central regulators for heart and renal function, angiotensin II and enkephalin, which trigger organ malperfusion and death in 20% of patients admitted to the ICU.
Professor Alexandre Mebazaa from Hôpital Lariboisie?re in Paris just in August published preclinical and clinical data demonstrating that DPP3-induced symptoms such as a significant reduction in the heart shortening fraction can be corrected by application of the anti-DPP3 antibody procizumab, developed by 4TEEN4 Pharmaceuticals GmbH, in mice with heart failure and that high DPP3 levels were correlated with worst outcomes – organ dysfunction and mortality – in humans with cardiogenic shock. In Berlin, Mebazaa backed the postulated mechanism showing preliminary data from patients with septic shock. As physicians have long been looking for markers of cell necrosis because they knew that’s why the patient dies in the end, many experts at the meeting were enthusiastic about DPP3, because such a marker would be useful for their daily routine and disease management of patients. An IB10 DPP3 POC test has been launched at the end of August for further clinical evaluation of DPP3 and R&D within the critical care community.
A second cause of sepsis mortality is kidney dysfunction. About 10% of patients admitted to the ICU – about 700,000 per year – die from worsening of kidney function, that causes acute kidney injury (AKI). SphingoTec said it expects to launch a CE-IVD POC test for penKid in January. PenKid is a quasi-real-time biomarker of kidney function, which seems to predict AKI up to 48 hours before onset, according to a broad body of observational data and new data presented by Professor Peter Pickkers (Radboud University, Nijmegen, The Netherlands) at the scientific symposium. Recent publications demonstrate that penKid identifies patients running into AKI with a single measurement at admission to the ICU/ED and that penKid can be used to monitor kidney function and efficacy of treatment. In order to directly compare the performance of the new penKid biomarker with the standard surrogate test of glomerular filtration rate (GFR), serum creatinine. In order to directly compare the performance of the new penKid biomarker with the standard test of estimated glomerular filtration rate (GFR) by serum creatinine, Pickkers is currently evaluating a new formula for GFR based on clearance of iohexol. The reason why we do so is that people used to look at GFR values for the diagnostic standard serum creatinine in ml/min but they are not very familiar with [how] concentrations of novel kidney function markers such as pro-enkephalin (penKid®) [correlate with functional GFR]. Once we have established such a formula linking penKid plasma concentration to GFR in ml/min, it will be easier to compare serum creatinine clearance performance with those of penKid and other biomarkers. We have two cohorts now for validation of this approach within some months in time," Pickkers said, adding, actually, we are collecting serial biomarker data from over 700 patients admitted to the ICU. Provided we find a bump in penKid level, say one day prior to the increase in creatinine in patients, that would be the proof that penKid provides a big advantage. This would allow the identification of the patient population who requires earlier therapeutic intervention, or secondary prevention to prevent the patient from further kidney injury."
The third cause of sepsis mortality identified within the Medicine4Future Initiative – the disruption in endothelial barrier function – affects most patients, almost 60%, according to Bergmann. SphingoTec’s biomarker bioactive adrenomedullin (bio-ADM) is a measure for endothelial barrier function. Disruption of endothelial barrier function, shown by high bio-ADM plasma levels, triggers oxidative cell stress, tissue edema and activation of the coagulation cascade, which together promote organ dysfunction and shock, reported Jesus Bermejo-Martin from University Hospital Rio Hortega Valladolid in Salamanca, Spain. High bio-ADM levels predict occurrence of septic shock 24 to 48 hours before onset as shown by observational studies on more than 20,000 patients and new data shown at the Berlin meeting. SphingoTec is expected to launch a POC test by mid-2020.
Professor Pierre-François Laterre from St. Luc Hospital in Brussels showed data in Berlin, which qualify bio-ADM testing as measure to add information to standard lactate monitoring of sepsis severity, that can be used to reclassify patient populations earlier and more specifically according to outcome. From multiple prospective studies we know that bio- ADM alters endothelial permeability and predicts outcomes in patients admitted with sepsis to the ICU and ED. However, when we will announce the results of the AdrenOSS-2 study in March 2020, which is about safety, tolerability and efficacy of the bio-ADM-targeting antibody Adrecizumab (developed by Adrenomed AG), we will have the proof that there is more than a correlation between bio-ADM on endothelial function and outcomes, Laterre told European Biotechnology. In this case, I presume, we will see becoming bio-ADM the diagnostic standard-of-care within only a few years of time because, as with PCT, physicians will be able to stratify patients according to the need for early aggressive intervention at admission of the intensive care unit or emergency department. According to Laterre, early risk stratification would safe significant amounts of cost. To evaluate if IB10 POC testing of bio-ADM it is cost-effective compared to the extremely cheap diagnostic standard lactate one must know the price per assay, Laterre told European Biotechnology.
As all three innovative biomarkers developed within the Medicine4Future Initiative and commercialized by SphingoTec predict outcomes in critically ill patients, physicians at EDs and ICUs can stratify patients who need a more aggressive treatment right from the start, said Laterre.
Experts at the meeting applauded the open innovation approach followed by SphingoTec. They provide us with the tests and equipment and let us play in order to find out the most useful applications for the biomarkers in intensive care, said Pickkers. Laterre underlined that if Adrenomed’s bio-ADM-targeted antibody adrecizumab, significantly reduced mortality in bio-ADM stratified patients with early septic shock no one in the critical care community will be reluctant to the innovative biomarker as this would be the definitive proof of clinical utility.
Fighting mortality is our ultimate goal, Bergmann underlined at the symposium. Together with the critical care community, we are working to provide diagnostic and therapeutic tools that provide personalised sepsis care with high clinical utility. We aim to start with POC tests but we are also open for collaboration with central lab testing providers to make our novel biomarker tests broadly available.


 Unsplash+
Unsplash+
