Incyte licenses Morphosys’ tafasitamab in US$2bn deal
Incyte Corp. has signed a global license agreement for commercialisation of Morphosys AG's anti-CD-19 programme tafasitamab.
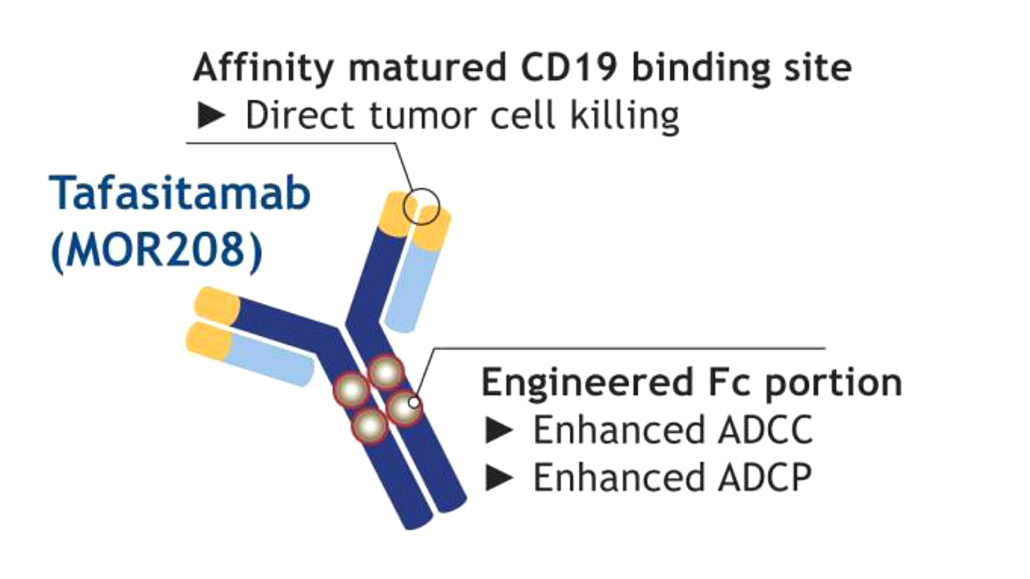
Tafasitamab (MOR208, Xmab5574) is an Fc-engineered antibody originally developed at Xencor, Inc. that targets CD19. The antibody is in clinical development for the treatment of B cell malignancies such as diffuse large B cell lymphoma (DLBCL, Phase II/III) and chronic lymphocytic leukemia (CLL, Phase II)
Under the agreemant, MorphoSys AG will receive an upfront payment of $750m from Incyte Corp. Additionally, Incyte will make an equity investment into MorphoSys of $150m in new American Depositary Shares (ADS) of MorphoSys. MorphoSys will be eligible to receive milestone payments amounting to up to $1.1bn and tiered royalties on ex-U.S. net sales of tafasitamab in a mid-teens to mid-twenties percentage range of net sales.
In the US, where tafasitamab got FDA breakthrough status and was filed for approval in December 2019, MorphoSys and Incyte will co-commercialize tafasitamab, with MorphoSys leading the commercialization strategy and booking all revenues from sales of tafasitamab. Incyte and MorphoSys will be jointly responsible for commercialization activities in the US and will share profits and losses on a 50:50 basis. Outside the U.S., Incyte will have exclusive commercialization rights, and will lead the commercialization strategy and book all revenues from sales of tafasitamab, paying MorphoSys royalties on ex-U.S. net sales. MorphoSys expects to complete an MAA to the EMA by mid-2020.
The companies will share development costs associated with global and US-specific trials at a rate of 55% (Incyte) and 45% (MorphoSys); Incyte will cover 100% of the future development costs for trials that are specific to ex-U.S.countries.
The agreement between MorphoSys and Incyte is subject to clearance by the US, German and Austrian antitrust authorities, and will become effective as soon as these conditions have been met.


 ©FabienMalot
©FabienMalot Lonza Group
Lonza Group Vetter Pharma
Vetter Pharma