EU diagnostic labs prepared for Sars-CoV-2
Shortly before a EU health ministers discuss the spread of the Chinese coronavirus, EU diagnostic labs have considered themselves prepared for a possible Sars-CoV-2 epidemic.
According to a survey carried out by the European Centre for Disease Prevention and Control (ECDC) and published in Eurosurveillance, 38 of 47 laboratories stated a capacity of at least 8,275 diagnostic tests per week. This seems enough for the moment, as human-to-human transmission i.e. through travelling have been reported in infectiosity clusters. However, the coronavirus’ contagiosity is still under discussion. However, if the number of infected people within the EU will increase exponentially, the test capacities in the central laboratories would have to be increased rapidly, the ECDC concluded.
According to the responses evaluated by Chantal Reusken from the Rijksinstituut voor Volksgezondheid en Milieu in Bilthoven and staff, 24 of 30 countries in the European Economic Area (EEA) were able to carry out virus detection tests at the end of January. The remaining 6 countries, including Poland and the Baltic States, said they would catch up until mid-Februar.
At the laboratory level, 38 out of 47 were able to detect the genes of Sars-CoV-2 in samples. A total of 19 laboratories were able to sequence the genome completely, another 15 laboratories were able to partly sequence the entire Sars-CoV-2 DNA. At the time of the ECDC publication, there were 36 confirmed infections in the EU.
At the industry side, French Novacyt Group (Paris) said that its UK-based molecular diagnostics unit Primerdesign Ltd (Camberley) has received the EU CE mark for the very first clinical PCR-based assay (COVID-19) able to detect the coronavirus. It can be used directly by laboratories and hospitals for the testing of patients without the need for validation by clinicians. Previously, the company had launched a research-only test. Primerdesign has already received requests for quotations for 288,000 CE-marked tests since they were made available to pre-order on 14 February 2020, mainly, from China, the US and the UK.
The Primerdesign test is being formally evaluated by public health authorities from five countries and the company is in discussions to potentially support national screening requirements for COVID-19.


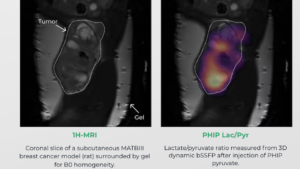 Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany
Courtesy Luca Nagel, Department of Nuclear Medicine, Klinikum rechts der Isar, School of Medicine, Technical University of Munich, Munich, Germany Sitryx Therapeutics
Sitryx Therapeutics