APEIRON Biologics licences MaxCyte’s electroporation platform
Viennese APEIRON Biologics and MaxCyte Inc have entered into licensing agreement covering the use of Maxcyte's electroporation platform for APN401 development.
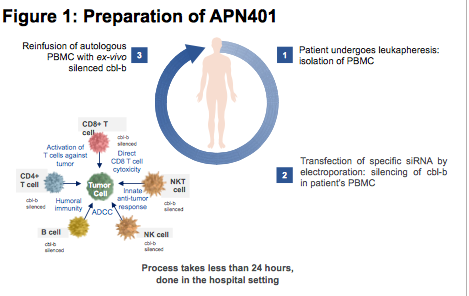
Under the agreement, APEIRON Biologics AG (Vienna) gets the right to utilise MaxCyte Inc‘s ExPERT® platform for the scaleable transfection of peripheral blood mononuclear cells (PBMCs) with siRNA directed against the immune checkpoint Casitas-B-lineage lymphoma protein-b (Cbl-b), an E3 ubiquitin ligase. Cbl-b has been shown to enhance T cell and natural killer (NK) cell-mediated antitumour activity in mouse tumour models. APEIRON Biologics licenced the technology targeting Cbl-b from the Columbia University and the Medical University of Innsbruck in 2018.
APEIRON’s cell therapy candidate, code-named APN-401, is currently in Phase Ib development as an experimental treatment for end-stage patients with certain solid tumours. In Phase I tests, APN401 did not show immediate severe side effects and was well tolerated using a dose of 50 x 105 PBMCs/kg. Acccording to APEIRON, targeting Cbl-b offers the opportunity to override numerous relevant checkpoints including sensitivity to regulatory T cells (Tregs), suppression by TGF-?, and immune regulation by CTLA-4 and PD-L1/PD-1 pathways.
MaxCyte’ Inc’s technology will help APEIRON Biologics to scale up cell transfection and production for manufacturing clinical lots of APN401. Under the terms of the agreement, the Viennese company obtains non-exclusive clinical and commercial rights to use MaxCyte’s Flow Electroporation® technology and ExPERT platform for the development of APN401. MaxCyte will receive undisclosed development and approval milestones and sales-based payments in addition to other licensing fees.
Through a capital increase in May 2020, APEIRON Biologics AG has received funds in the double-digit million range for the development of its Phase II COVID-19 medicine APN01 and APN401.


 ©FabienMalot
©FabienMalot Lonza Group
Lonza Group Vetter Pharma
Vetter Pharma