Novartis AG executes licence option with Molecular Partners
After Molecular Partners AG reported an 78% reduction of hospitalisation by a single ensovibep dose in infected COVID-19 patients, Novartis AG is to pay a CHF150m milestone.
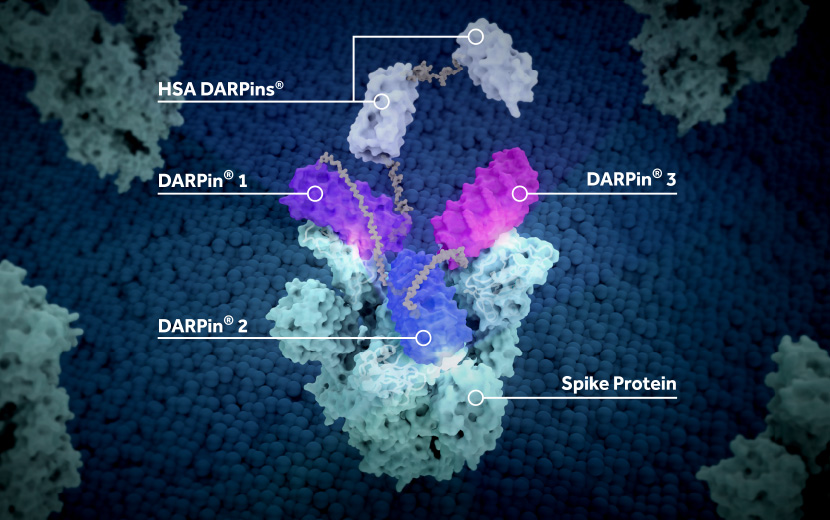
Further results from the randomized, placebo-controlled Phase IIa EMPATHY trial in acute COVID-19 ambulatory patients suggest that Molecular Partners’ DARPin (Designed Ankyrin Repeat Protein) antiviral therapeutic candidate that bind at three different sites of the viral spike protein met the primary endpoint of viral load reduction over eight days. Besides the 78% reduction in hospitalisation compared to placebo in the target population, no deaths were observed in the ensovibep treatment arms (75mg, 225mg and 600mg) of the dose-finding study. Molecular Partners and its licence partner Novartis AG plan to work with 75mg for further development. Phase IIb results are expected in the first half of 2022.
Ensovibep (MP042) is a small molecule construct with antibody-like binding affinity that simultaneously binds to three sites on the receptor-binding domain (RBD) of the spike protein of SARS-CoV-2 to prevent the virus from entering cells. In a previous clinical study funded within the US ACTIV3 programme by the NIH in hospitalised COVID-19 patients, ensovibep failed to clear the efficacy bar, leaving hopes for ensovibep resting on the ongoing separate trial in non-hospitalised patients. While antibody treatments from Brii Biosciences, Eli Lilly and the GlaxoSmithKline-Vir Biotechnology collaboration all failed, the hypothesis was cemented that drugs that act on the virus itself are ineffective in hospitalized COVID-19 patients.
The companies reported that ensovibep maintained potent in vitro pan-variant activity against all variants of concern identified so far, including Omicron. Novartis confirmed it will exercise its option to in-license ensovibep from Molecular Partners, accelerate manufacturing scale-up, and plans to seek expedited regulatory authorizations globally – first via the US Food and Drug Administration’s (FDA) Emergency Use Authorization (EUA). Upon completion of in-licensing, Molecular Partners will receive a milestone payment of 150M CHF and be entitled to a 22% royalty on sales of ensovibep in commercial territories.
The global EMPATHY clinical trial, which is being conducted by Novartis, with Molecular Partners as sponsor, is a randomized, double-blind, placebo controlled study in ambulatory (non-hospitalized) adult patients with COVID-19. EMPATHY Part A enrolled 407 patients to identify a dose of ensovibep with optimal safety and efficacy. In the 301 patients treated with ensovibep, there were four events; hospitalizations occurred in two patients and two needed to visit ER (event rate of 1.3%).


 ©FabienMalot
©FabienMalot Lonza Group
Lonza Group Vetter Pharma
Vetter Pharma