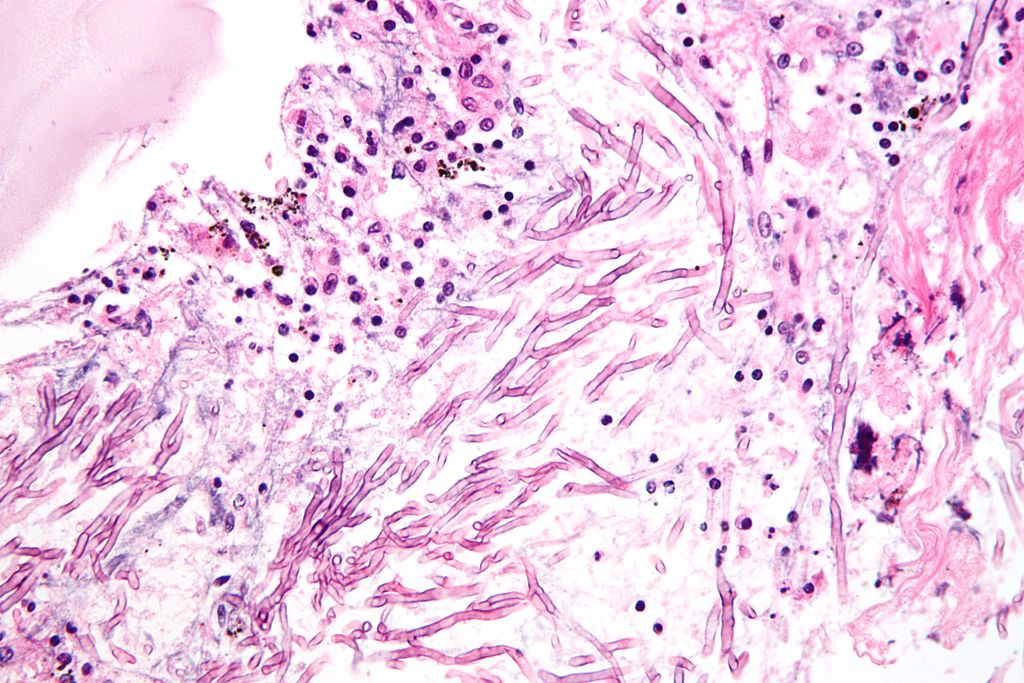
F2G to use $70m financing to advance olorofilm
British antifungal developer F2G Ltd has announced a $70mn financing round to push development of its late-stage compound olorofilm.
The financing was co-led by new investors Forbion and Sofinnova Partners. Existing investors, Novo Holdings, Morningside Ventures, Cowen Healthcare Investments and Advent Life Sciences also participated in the funding round. The proceeds come on top of a recent $480m licence agreement with Shionogi & Co., Ltd., and will enable F2G to advance late-stage development and commercialisation in the US of olorofim, a novel oral antifungal therapy to treat invasive aspergillosis and other rare mold infections. Nanna Lüneborg of Forbion and Joe Anderson of Sofinnova Partners will join the F2G Board of Directors.
Olorofim represents the first novel class of antifungals developed in the past 20 years and is the only antifungal medication to be awarded a Breakthrough Therapy Designation for multiple indications by the US Food and Drug Administration (FDA). Olorofim works through a unique mechanism of action, different from existing classes of antifungals, exerting fungicidal activity through inhibition of the pyrimidine synthesis pathway. It is anticipated to be used to treat patients with a serious invasive, rare fungal disease where existing treatments are inappropriate or no longer effective. Data from F2G’s open Phase 2b study in patients with rare and resistant molds, who have limited treatment options, will be presented at ID Week annual conference in October.
In May 2022, F2G entered a $480m strategic collaboration with Shionogi & Co., Ltd. to develop and commercialize olorofim in Europe and Asia which included $100m in upfront payment and $380m in regulatory and commercialization milestones plus double-digit royalties on sales.




