Adrenomed set to start pivotal trial in sepsis
German Adrenomed AG has got a recommendation from the EMA to push its Adrenomedullin-binding antibody Adrecizumab into a pivotal Phase III study.
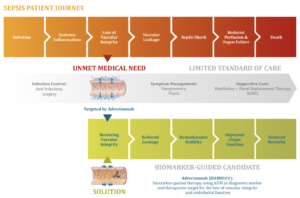
The vascular medicine specialist Adrenomed AG (Hennigsdorf) has received positive feedback from the European Medicines Agency (EMA) on the intended marketing authorisation study for Adrecizumab (Enibarcimab). The first-in-class antibody, which binds the peptide hormone adrenomedullin, eliminates dangerous inflammation-related vascular leakage in patients with early septic shock and, according to data to date, reduces the resulting leakage of fluid into the tissue, subsequent drop in blood pressure and resulting multi-organ failure due to insufficient blood supply.
So far, there are only insufficient treatment options for septic shock. The EMA recommends continuing the development of adrecizumab based on the completed Phase II AdrenOSS-2 study, which showed a statistically non-significant but positive survival trend. In the planned pivotal trial, intensive care patients with early sepsis will be stratified based on the blood concentration of the surrogate biomarker bio-ADM and given standard therapy in the placebo arm. In the verum arm, they will additionally receive the vasculature-stabilising antibody adrecizumab. The primary efficacy endpoint is mortality according to the EMA guideline for the treatment of sepsis. The study results serve as the basis for the application for marketing authorisation of adrecizumab.


 SANOFI
SANOFI