Learning to listen to your gut
The adage you are what you eat' is more than folk wisdom. Evidence that the food you ingest has a major impact on your health grows by the day. It's now clear the bacteria that colonise the skin and gut from birth on are key sensors for environmental triggers in chronic diseases of civilisation. Ever since research showed that restoring microbiome balance cures 90% of all Clostridium difficile infections, food manufacturers, pharma companies and independent teams have been tracking it in other indications - including multiple sclerosis.
When Dr. Terry Wahls was diagnosed with remitting-relapsing multiple sclerosis (MS) in 2000, she did what you would expect a professor for internal medicine at the University of Iowa to do. She began taking one of the ABC’ medicines (Avonex/Betaseron/Copaxone) – compounds to suppress the autoimmune reaction in her Central Nervous System (CNS) that was targeting the myelin insulation that covered her neurons – and hoped for the best. When she ended up in a tilt-recline wheelchair three years later after developing the untreatable secondary progressive form of MS, she decided she had to find a way out on her own. The strictly science-driven expert for clinical trials began to comb scientific literature for alternatives to mainstream immuno-suppressive drugs , which are able to slow but not cure her neurodegenerative disorder. She ended up conducting experiments on herself.
Through a combination of electrostimulation and a paleo-like’ menu that cut down on gluten-rich foods and increased non-processed foods in her diet, the physician was back on her own feet in a year. She also no longer suffered from the heat intolerance and fatigue that are top causes of MS disability. Inspired by the recovery, she set up a foundation that eventually attracted 90,000 followers, then wrote a book to bring her message to the 2.5 million people who suffer from MS globally. In it she says, medication can’t take away your autoimmune disease, but your body can heal itself.
Probiotics are the golden goal
Interestingly, the approach the physician takes to treating the neurodegenerative disease through the Wahls Protocol’ comes at a time when public and science are ready to listen. Around 70% of European consumers now say they believe healthy food positively impacts on health. A huge number of scientific publications have also linked the composition of an individual’s gut bacteria, or microbiome, to susceptibility for chronic diseases as different as diabetes, autism, chronic bacterial infections, cancer, Crohn’s disease, psoriasis, arthritis – and multiple sclerosis. When studies showed that fecal microbial transplants (FMT) can cure recurrent infections with antibiotic-resistant Clostridium difficile bacteria in nine out of ten cases, researchers and companies were hit with gold rush fever. A new area of endeavour was thrown open; it’s aimed at understanding which factors in the microbiome interact with the body to trigger diseases that have a feature in common – chronic, low-threshold inflammation.
As imbalances in the microbiome – scientifically dubbed dysbiosis’ or dysbacteriosis’ – are clearly dependent on what you eat, food companies have entered the space along with pharma firms. While AstraZeneca and Merck, Sharp & Dohme, and DuPont Nutrition have all installed large microbiome research centres and joint ventures in Europe, other big players are trying to cherry-pick best approaches from a growing number of microbiome SMEs . In December 2015, Swiss pharma major Novartis and global food giant Danoneacquired interests in Seventure’s €160m Health for Capital Life fund, the only VC fund so far dedicated exclusively to investments in microbiome-related companies. The next five years will see the first microbiome products coming out of clinical studies and onto the markets, says Seventure partner Eric de la Fortelle. We already have some products in Phase IIb and III. These will become medical or nutritional products, and the range of indications will keep expanding, he believes. Analysts agree. They predict the microbiome market could become as big as biotech in the next 20 years. One projects a market value of US$658m for microbiome-derived medicines by 2030.
No microbiome = No MS
There’s huge hype around microbiome research. In the past two years, vast amounts of money have been channeled into the field, says Christine Lang, CEO of Novozymes subsidiary Organobalance, a specialist for lactobacterial interventions. Last year, about US$700m were invested in the US and €600m in Europe. I’m convinced that we’re currently just seeing the tip of the iceberg. However, understanding of the microbiome is early-stage, and the next step is to investigate what happens in the gut mechanistically. Nobody currently knows whether microbial products or an accumulation of certain bacterial species triggers disease, she adds.
To date the only commercial provider of germ-free mice and customised microbiome solutions globally, Taconic Biosciences has reported a growing interest from a wide range of for-profit and non-profit groups. Demand has expanded exponentially in the last few years, says Randi Lundberg, the head of the firm’s Field Applications Scientist Microbiome Products & Services. Back in 2011, a mouse model proved beyond doubt that MS genesis is tied to microbiome developments. We know more than 200 genes that make people susceptible to multiple sclerosis, says Hartmut Wekerle, a top MS researcher at the Munich-based Max Planck Institute of Neurobiology. For onset, however, it needs a trigger. Since the digestive tract is actually the most intimate connection between the outside world and the immune system, his team investigated whether the microbiome could play the role of trigger in immune-related conditions and environment. But to find that out, they had to solve another problem first. Current models of experimental autoimmune encephalitis (EAE) didn’t work for that purpose, because they externally supply the mice with the human myelin protein to induce brain inflammation. So we designed a model that mimicked many aspects of spontaneously developing MS. Our mice express a transgenic T cell receptor that detects myelin autoantigens, and makes the animals develop EAE spontaneously after 6-8 weeks. Using this model, Wekerle’s team proved that MS cannot develop in the absence of the microbiome. When we reared such mice in a germ-free environment, they didn’t develop EAE. When we transplanted a microbiome into them, they did, Wekerle explains. This was the ultimate proof that a factor linked to the microbiome triggered MS in animals. We hypothesise that the gut microbiome pushes the intial activation of autoreactive T cells, which might initiate MS. If this hypothesis holds true in humans, microbiome-derived pre- or probiotics, defined fecal microbial transplants or therapies that correct dysbiosis could in the future completely transform the current US$22bn MS market.
Searching for the nuggets
Since Wekerle’s groundbreaking study, many teams have begun classifying disease-relevance of gut bacteria in MS patients according to abundance – with limited success. A very recent study carried out by Wekerle’s US partner group led by Sergio Baranzini at UC San Francisco, however, is the first to add functional relevance to the data flood. Investigating the microbiomes of 71 MS patients and 71 healthy volunteers, the US team found that two bacteria species enriched in the MS group vs. controls – Akkermansia muciniphila and Acinetobacter calcoaceticus – triggered immune cells in vitro to become pro-inflammatory. At the same time, a species that was reduced in MS patients (Parabaxteroides distasonis) boosted immune-dampening responses. When transplanted into germ-free mice that had no microbiome, lead author Egle Cekanaviciute found the same effect. To investigate how the differences in gut bacteria modulate the immune system’s attack on myelin, the researchers performed fecal transplants on mice with induced EAE. Analyses revealed that mice carrying the microbiome of MS patients caused them to lose regulatory T cells, to produce less of the immune-regulatory messenger interleukin-10, and to develop more serious neurodegeneration. It looks like these microorganisms could be making the disease progression worse or better, Cekanaviciute interprets. If proven safe and effective, a fecal microbial transplant could be a first-line option for many patients, says Baranzini. We’re working to get IND approval from the FDA, and expect to start a Phase I trial early next year. In collaboration with the International Microbiome Study (IMSMS), a consortium of leading US, German, Spanish, UK and Argentine MS research teams, founder Baranzini will recruit 2,000 patients with all forms of MS/MS treatments and 2,000 healthy controls to systematically compare differences in the microbiota or its physiology that could be linked to MS.
Also in October, Wekerle and clinical partner Reinhard Hohlfeld, Director at the Institute for Clinical Neuroimmunology in Munich, examined whether findings in mice also apply to humans. To this end, they recruited 34 identical twins, each with one diseased and one non-affected sibling, and transplanted their respective intestinal microbiomes into germ-free mice that mimick the spontaneous development of MS. When the stool of MS-affected twins was transplanted, we observed the spontaneous development of the disease significantly more often and more pronounced, says Hohlfeld. The German researchers next want to find out whether the activation of the autoimmune reaction against the myelin protein in MS patients is set in motion by bacteria in the small intestine, which most often trigger pro-inflammatory responses, or whether it occurs in the large intestine, where immune-regulatory processes are dominant. Their first results, which have yet to be confirmed in further studies, seem to corroborate the Baranzini team findings: that reduced immunoregulatory activity in MS patients might trigger the activation of autoreactive T cells that marks the onset of MS.
Diet vs. fecal transplants
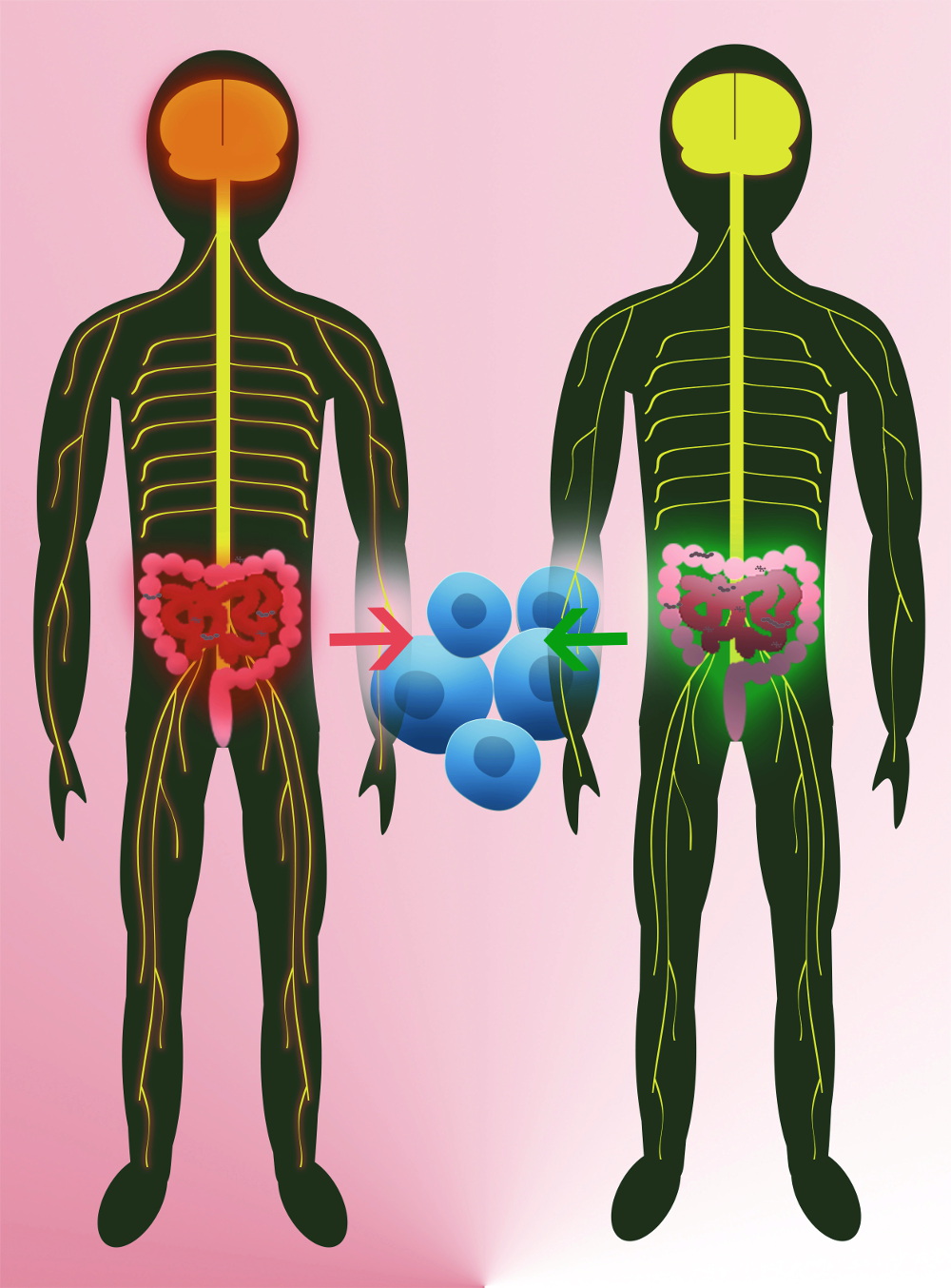 Wekerle hopes his work will encourage not only probiotic therapies, but also non-interventional approaches to the effects of nutrition on MS. While the golden goal of probiotic or dietetic therapy is still a long way off, he adds, FMT is also difficult. Nobody currently knows what makes a healthy fecal transplant. Additionally, a lot of obscure providers are offering wild transplants. It’s essential for the safety of patients to distiguish serious researchers from that groups.
Wekerle hopes his work will encourage not only probiotic therapies, but also non-interventional approaches to the effects of nutrition on MS. While the golden goal of probiotic or dietetic therapy is still a long way off, he adds, FMT is also difficult. Nobody currently knows what makes a healthy fecal transplant. Additionally, a lot of obscure providers are offering wild transplants. It’s essential for the safety of patients to distiguish serious researchers from that groups.
The new field has also seen setbacks. Published 12-month results of an openlabel clinical pilot study enrolling 20 MS patients to prove efficacy of the Wahls Protocol reported only partial success. While patients with moderate motor defects benefited, those with severe disability didn’t respond (Degen Neurol Neuromuscular Disease, doi: 10.2147/DNND.S128872). At the end of October, however, a team of Scottish, US and French researchers added further insight into the potentially critical immune regulatory pathway that is defective in patients with MS. Led by Siobhan Ni Choileain from the University of Edinburgh, the team reported that an altered glycosylation pattern of the CD46 protein blocked TH1 cells from properly developing into IL-10-secreting type 1 regulatory T (Tr1) cells, which tamp down inflammation and prevent autoimmunity (Science Signal., doi: 10.1126/scisignal.aah6163). Researchers will now be able to investigate microbiome impact on the newly identified trigger.
(First published in European Biotechnology, Winter Edition 2017)



 Microbiotica
Microbiotica Araris Biotech AG
Araris Biotech AG