Cutting time to market for high quality therapeutics
The demand for the fast and robust development of manufacturing cell lines is ever growing, with an increasing number of therapeutic proteins in development. To fulfill these needs, Celonic engineered the cell line kit CHOvolution, which equips users with everything required for the development of mammalian cell lines and provides an integrated support system for assistance.
The CHOvolution platform is based on a CHO-K1 host cell line and its proprietary SEFEX (SErum Free EXpression) technology platform to provide the best screening and selection processes. CHOvolution is adapted for creating robust and stable cell lines that express biologics in serum and protein-free suspension culture and commercially available media. This feature ensures regulatory compliance in numerous customer projects entering clinical development phases.
Reducing time to clinic with CHOvolution
 Bringing a drug from discovery to the marketplace remains a costly, complex, and time-consuming process, especially in high-stakes races such as to be first-to-market. Celonic recognises this and appreciates that improvements to major bottlenecks and reductions in timelines add up. For any drug developer or service provider, cell line development is one of the most challenging phases, especially when subsequent GMP compliance is required.
Bringing a drug from discovery to the marketplace remains a costly, complex, and time-consuming process, especially in high-stakes races such as to be first-to-market. Celonic recognises this and appreciates that improvements to major bottlenecks and reductions in timelines add up. For any drug developer or service provider, cell line development is one of the most challenging phases, especially when subsequent GMP compliance is required.
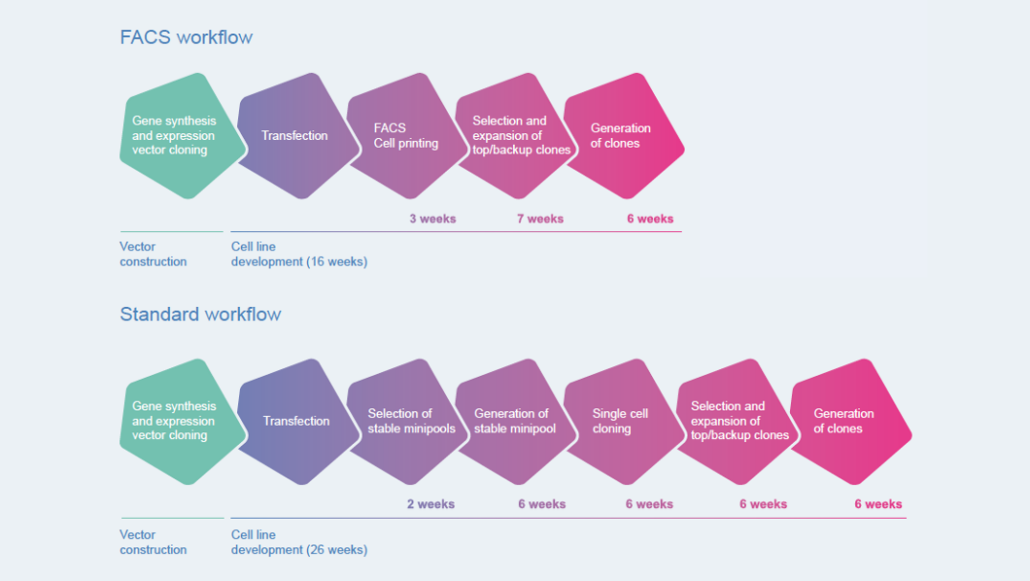
To enable the same excellent results within shortened timelines, Celonic invested in state-of-the-art technologies and optimised project workflows to create high-producing cell lines (Fig. 1). Successful implementation of technologies such as FACSAria® Fusion cytometer and cell printing have facilitated fast track workflow options for high priority projects, bringing the entire timeline from biological sequence to GMP-compliant material from 18 months down to approximately 14 months.
Scalable high-titer production of therapeutic proteins
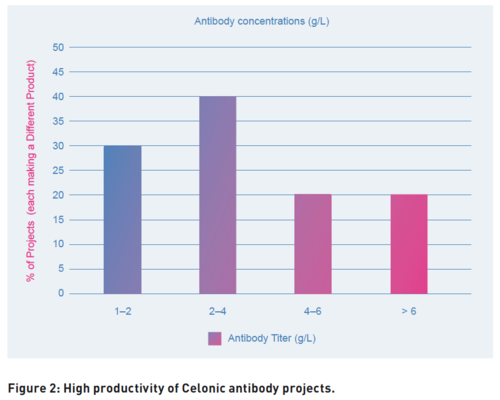
 At Celonic, CHOvolution forms the workhorse for mAb production and demonstrates high titers (up to 8 g/L) with the median productivity of a CHOvolution cell line in the 2-4 g/L range (Fig. 2).
At Celonic, CHOvolution forms the workhorse for mAb production and demonstrates high titers (up to 8 g/L) with the median productivity of a CHOvolution cell line in the 2-4 g/L range (Fig. 2).
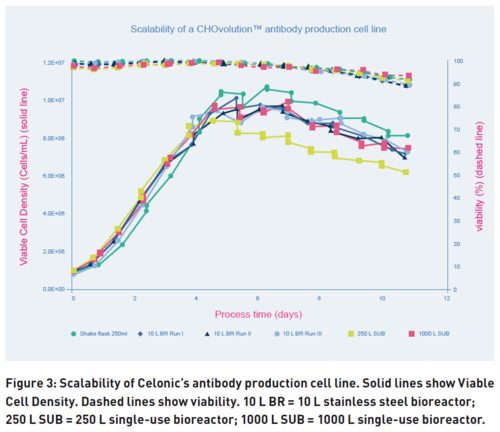
One of the most important characteristic of any cell line is its scalability. Effects such as increased generation cycles and the subtle differences in the upscaling of processes can often accumulate and sometimes result in dramatic losses in productivity. CHOvolution cell lines, tested up to 100 generation cycles, retain their genetic stability, which allows the cells used for development to be used in commercial manufacturing. Combining this stability and in-house expertise results in processes with CHOvolution being predictably scalable and displaying almost identical behaviors from the shake flask to 1,000 L bioreactor scales (Fig. 3).
This combination of a fully scalable cell line with high titers that can be developed in a competitive time-frame makes CHOvolution a powerful tool in the drug developer’s arsenal.
CHOvolutionTM licensing
Since July 2015, Celonic has been licensing its CHOvolution platform to drug developers and to other service providers. The license package includes the host cells, vector set, and detailed protocols for the handling, screening, and selection processes necessary to generate high-performing production cell lines. The license is royalty-free and with a guarantee to licensees: we will take over the developed cell line from our licensees and upgrade it to a GMP compliant Master Cell Bank.
The CHOvolution is a proven production cell line technology for:
- antibodies of different isotypes (e.g. IgG1, IgG2, IgG4);
- bispecific and single-chain antibodies;
- enzymes, growth factors, and hormones.
Key features include:
- cell line adapted to serum-free EMA and FDA-compliant media;
- high productivity (up to 8 g/L for mABs);
- high scale-up stability (over the 120 generation cycles and easily scalable from shake flask to 1000L volume);
- GMP-compliant, from R&D to market manufacturing;
- tailored workshops and hands-on training to develop the product;
- technical and scientific support on the CHOvolution platform.
Celonic is a privately owned CDMO based in Basel, Switzerland. The company provides a range of services from cell line engineering and process development to cGMP manufacturing for new biological entities (NBEs) and biosimiliars worldwide. Celonic’s ethos focuses on applying empathy, efficiency, and excellence in all business aspects to ensure its clients attain their goals more efficiently and reliably.




