Innovative delivery: dual-chamber systems
As the global demand for injectables grows, so does the demand for innovative delivery systems. For lyophilised forms, dual-chamber systems offer advantages. They have been on the market since the mid-1980s, mainly for emergency or chronic medication. The systems have been developed for the convenience of the patients/caregivers, but they also offer benefits for the pharma/biotech companies in regards to low residual volume and increased API yield.
When considering lyophilisation in a dual-chamber system, it is important to understand the development process. A five-step approach can help determine whether it is a viable option for an injectable drug.
Step-by-step approach
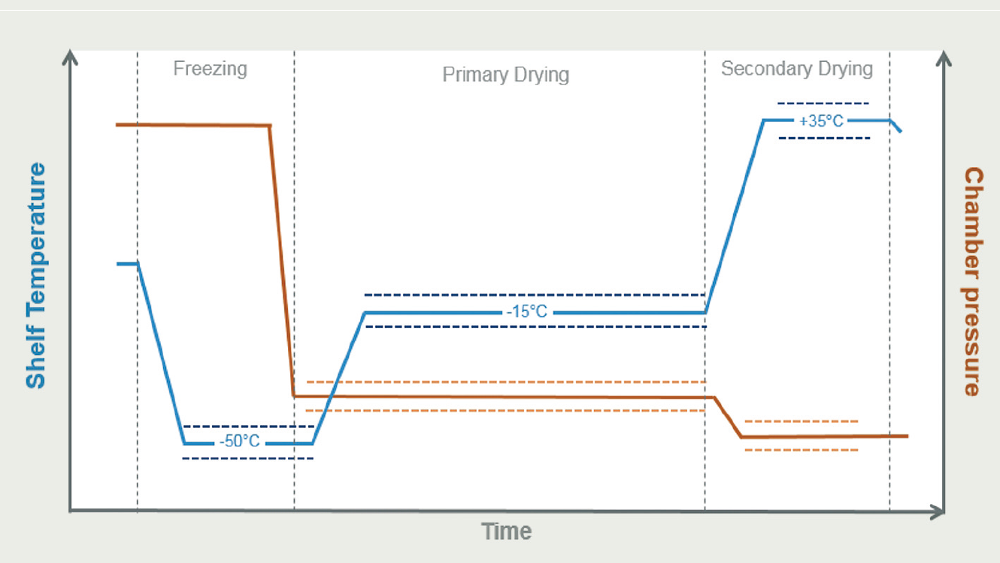
The first step is to determine compatibility utilising a series of lyophilisation cycle feasibility studies. Trials based on existing vial lyophilisation development are performed, as are concentration/ fill volume studies and cycle options to test the product viability. The drying process is governed primarily by convection and radiation rather than direct heat conduction and needs to be adjusted accordingly.
The second step focusses on process characterisation studies. An assessment of the current process and a variety of compounding, filtration, and pumping tests provide guidance for further studies required for the development in a dual-chamber system to identify suitable settings for accurate dosing.
The third step includes design of experiment (DoE) cycle development and robustness. This is carried out with varying temperature and pressure combinations to test the design space limits (edge of failure) for both primary and secondary drying. Visual appearance of the lyo-cake, reconstitution times and residual moisture, and further critical quality attributes such as potency, stability, and sterility are analysed.
The fourth step involves siliconisation and functionality testing. While necessary as a lubricant, silicone oil must be kept to a minimum and evenly distributed to maintain acceptable break-loose and glide forces. Closure integrity and stability tests are also performed with methods like dye-ingress or vacuum decay testing.
The fifth step entails engineering runs to ensure scalability. The first stage is a non-GMP commercial scale-up testing general feasibility. It also includes temperature mapping and sample analyses. The second stage concentrates on lyophilisation-cycle adaptation and testing. Trials with a variety of concentrations are performed under seeded run conditions. This allows for a minimisation of used drug substance while still obtaining a good indication of the performance. Finally, process qualification based on the results from development is performed.
In summation, dual-chamber systems offer an innovative delivery option for lyophilised drugs and can also extend the shelf-life of complex compounds. Taking the right approach is essential to determine if such a system is suitable for your compound.


 Beiersdorf
Beiersdorf Brainomix
Brainomix BioNTech SE
BioNTech SE