Radiant Precision
Precision Oncology High-Precision Radionuclide Therapy is a promising, new generation of targeted molecular therapy in which the smallest amounts of medical radioactivity find their way through the bloodstream specifically to the tumour cells for diagnosis and treatment of cancer.
Since the time of Marie Curie, radiation research has been fundamental for the development of nuclear medicine. Furthermore, biomedical investigations on various tumour markers have contributed to the evolution of Precision Oncology, paving the way for Targeted Radionuclide Therapy.
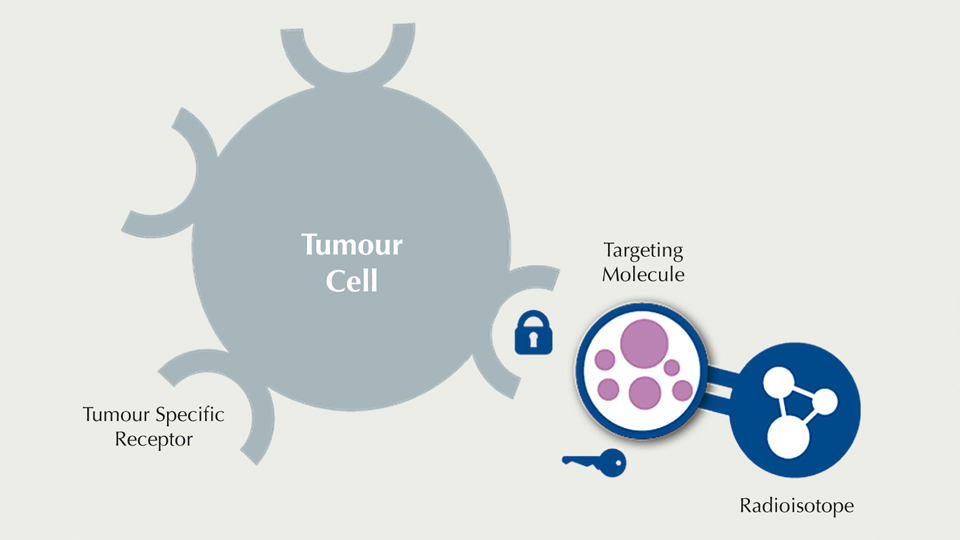
In contrast to radiotherapy, where radiation is applied from outside the body, Targeted Radionuclide Therapy is defined by the injection of a radiopharmaceutical, which precisely recognises tumour cells. Radiopharmaceuticals consist of a medical radioisotope conjugated to a tumour-specific targeting molecule that binds specifically to a tumour antigen according to the lock-and-key principle. A very small amount of medical radiation is sufficient for this therapeutic approach. Due to the efficacy and specificity of Targeted Radionuclide Therapy, healthy tissue is minimally affected, and side effects are maximally reduced.
In many cases, the targeting molecule can be used for both diagnosis and therapy, the only difference being their conjugated radioisotope. Radioisotopes with shorter half-lives are used for diagnosis, while therapeutic ones have slightly longer half-lives.
The biotechnology and radiopharmaceutical group of companies ITM Isotopen Technologien München AG (ITM) develops, produces, and globally supplies a new generation of radiopharmaceuticals for Targeted Radionuclide Therapy. ITM has established a method to produce the highly pure, no-carrier-added (n.c.a.) Lutetium-177, which does not contain impurities, with metastable, long-lived Lutetium-177m. This ensures economical waste management and environmental sustainability. In 2019, ITM received the German Medical Award for their dedicated work in this field and in 2020 the prestigious recognition of €40m from the European Investment Bank. ITM’s goal is to achieve a sustainable medical benefit with their therapeutic approach and to significantly improve treatment outcomes and quality of life for cancer patients.
How to improve outcomes?
We are currently conducting the clinical phase III trial COMPETE, which is investigating the efficacy and safety of the therapeutic candidate n.c.a. 177Lu-Edotreotide, says ITM’s CEO Steffen Schuster. n.c.a. 177Lu-Edotreotide is comprised of a somatostatin analogue as the targeting molecule and ITM’s highly pure n.c.a. Lutetium-177 and is used for the treatment of somatostatin-positive neuroendocrine tumours (NETs) of gastroenteropancreatic origin (GEP-NETs). NETs are rare diseases, thus exacting a high demand for effective therapies. We are confident that COMPETE will confirm the promising results of our Phase II study and will give many patients the opportunity to benefit from this new generation of Targeted Radionuclide Therapy, added Mr Schuster.
In addition, ITM is working on further product candidates for diagnosis and therapy of glioblastoma, osteosarcoma and bone metastases and other cancer indications, thereby actively shaping the progress of radiopharmaceutical research and development a radiant contribution to treating cancer.
This article was published in the European Biotechnology Magazine Autumn Edition 2020.



 eXmoor Pharma
eXmoor Pharma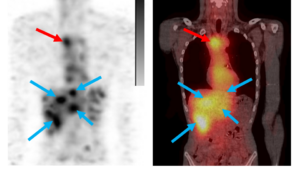 Molecular Partners AG & NuMeRI, presentation Feb. 2026
Molecular Partners AG & NuMeRI, presentation Feb. 2026