Targeting coronaviruses
Viennese APEIRON Biologics AG raised €17.5m to expand ongoing Phase II trials of its
coronavirus-specific drug candidate APN01 in Europe to the US and Russia. The recombinant angiotensin-converting enzyme 2 (rhACE2) mimics the receptor on the human cells that coronaviruses are using to infect these cell. Preclinical and clinical data demonstrate that APN01 is well-tolerated and can stop the overshooting host immune responses that are triggered by virus proliferation.
APN01 (rhACE2) is one of the most advanced drug candidates for the treatment of COVID-19 and one of the few therapy approaches specifically directed against the coronavirus. Originally, the recombinant human Angiotensin Converting Enzyme 2 (rhACE2) was designed by Apeiron to target SARS-CoV-1 during the first SARS epidemia. When SARS-CoV-1 disappeared APN01 was clinically developed for the treatment of acute lung injury (ALI), acute respiratory distress syndrome (ARDS), and pulmonary arterial hypertension. ALI/ARDS is the major source of Covid-19 mortalities.
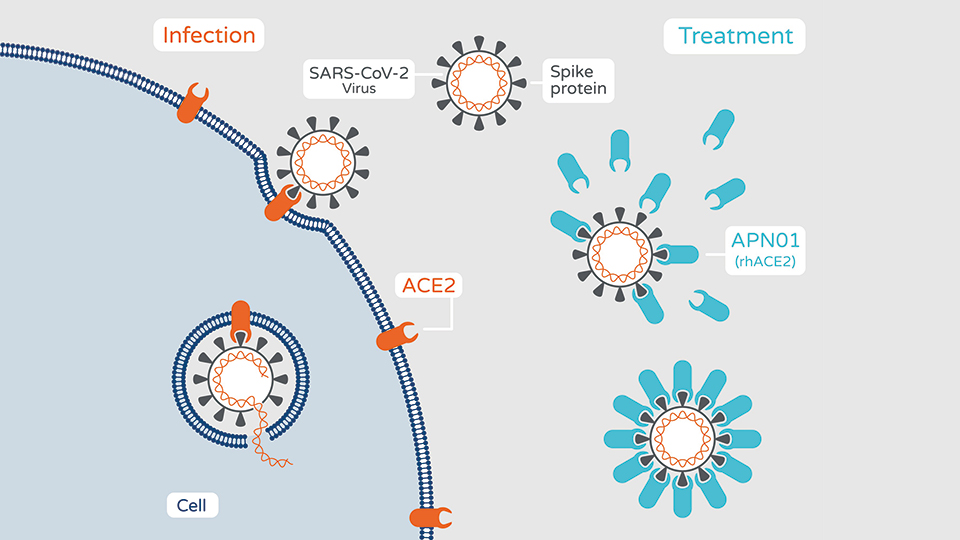
SARS and SARS-CoV-2 use the human ACE2 receptor, which is expressed in human airway epithelia as well as lung parenchyma, to enter human cells. Soluble APN01 binds specifically to the viral Spike (S-) protein and inhibits infection and protects the lung (see figure).
The double-blind, randomised, placebo-controlled study aims to treat 200 patients with severe COVID-19 disease to further data characterise the impact of rhACE2 on biological, physiologic, and clinical outcomes, as well as safety in patients with severe SARS-CoV-2 infection. If the current Phase II study is positive, regulatory agencies may grant accelerated market approval.
Advancing the clinical pipeline
At the beginning of June, APEIRON Biologics AG completed a financing round totalling €17.5m. As part of a rights issue with a private placement, the company was able to raise €11.9m from existing and new private and institutional investors, including the Vienna Insurance Group, which contributed approximately €7m, resulting in a participation of 3.26% in APEIRON Biologics. In addition, the Austrian Research Agency (FFG), the Vienna Business Agency (WAW), the Austria Economic Service Company (AWS), and Erste Bank have earmarked public funding and guarantees totalling €5.6m. The assets will be used to advance the development of APN01 and the further development of immuno-oncology projects."With the completion of these capital measures the financing of our clinical COVID-19 development and our immuno-oncological cell therapy projects is secured and the whole team at APEIRON is highly commited to drive both programs forward to bring new treatment options to patients in need," said Peter Llewellyn-Davies, CEO of APEIRON.
Contact
Apeiron Biologics AG
Dr. Peter Llewellyn-Davies, CEO
investors@apeiron-biologics.co
www.apeiron-biologics.com
This Advertorial was published in the European Biotechnology News Magazine Summer Edition 2020.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink