Antiviral drugs for pandemic preparedness
Viral pandemics are not a once-in-a-lifetime event but a constant threat looming over our heads. While the fight against SARS-CoV-2 is currently a top priority, we should not lose sight of what might come next. Broad-spectrum antiviral drugs like Atriva's ATR-002, currently in Phase II clinical development for treating COVID-19, could be an effective measure in the fight against the current pandemic and future viral outbreaks.
What was first the virus or the cell? To date, this question remains unanswered. What is clear is that viruses parasitic genetic material packaged in a shell or an envelope have been hijacking human cells ever since mankind came into existence. Unable to reproduce on their own, viruses take advantage of our sophisticated cellular machinery to make thousands of copies of themselves, killing our cells in the process. Our history together has been a troubled one: while many viruses are simply a nuisance, giving us a runny nose or a light fever, others cause severely disabling symptoms and even death.
Every once in a while, a new virus capable of infecting large numbers of people emerges, causing worldwide pandemics. This is not a recent phenomenon: The last century alone saw three major worldwide influenza outbreaks (1918, 1957 58, 1968) and the start of the HIV pandemic. So far, in the twenty-first century, there has been an influenza pandemic in 2009 and the current SARS-CoV-2 outbreak. And it does not stop there. Recent reports identify a new type of swine flu with pandemic potential.
Pandemic preparedness
We need to be prepared to fight these pandemics, so we do not have to suffer losses and halt our economies every few years because of a novel viral threat. While it is extremely important to develop treatments and vaccines for the current SARS-CoV-2 outbreak, we also need to think about the future, as pandemics are here to stay.
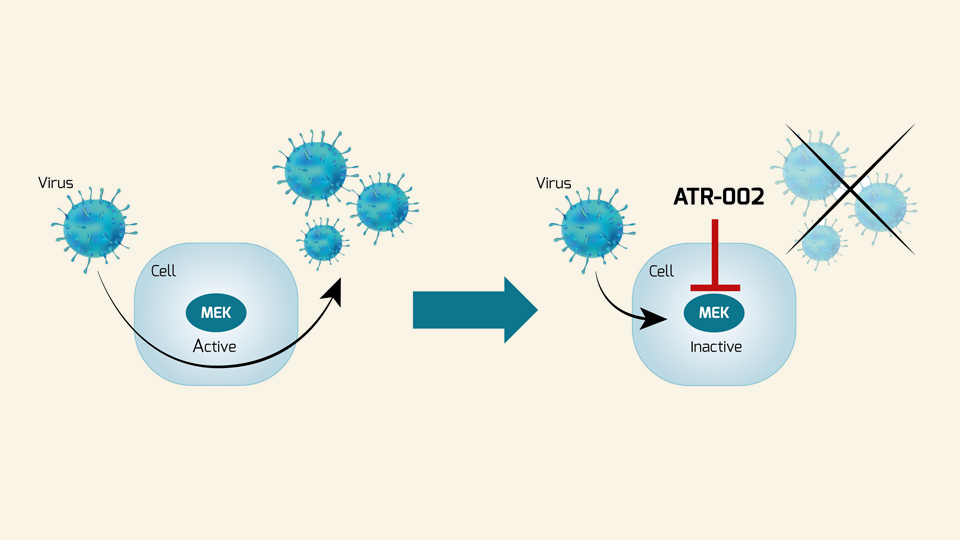
Once a new virus shows up, effective antivirals are needed to address it. The current toolbox against viruses contains some options; however, many are limited to a few specific viruses, have a small therapeutic window, and are susceptible to viral evasion. New approaches are needed to equip ourselves for future viral threats. Since 2015, Atriva has been working to develop novel antiviral therapies that function against a wide range of respiratory infections, with an initial focus on influenza. The company’s lead drug candidate is the MEK inhibitor ATR-002, an oral small molecule with a dual benefit directed against both viral replication and excessive host immune responses. Unlike other approaches, ATR-002 does not target the virus directly, but inhibits a host cellular pathway that is essential for the replication of a number of RNA viruses. This host-targeting approach makes the development of viral drug resistance far less likely and can combat any virus that relies on Raf/MEK/ERK signalling. Besides influenza virus and SARS-CoV-2, many other life-threatening respiratory RNA viruses take advantage of this pathway, including RSV and Hantavirus, which is associated with bio-weapon potential. ATR-002 is currently in clinical development for the treatment of COVID-19 (Phase II, ongoing) and influenza (Phase II planned for winter 2020/2021). If successful, it could become a unique broad-spectrum antiviral and the instrumental answer to viral pandemics and seasonal epidemic threats, such as influenza.
Contact
Atriva Therapeutics GmbH
info@atriva-therapeutics.com
www.atriva-therapeutics.com
This Advertorial was published in the European Biotechnology News Magazine Summer Edition 2020.


 Unsplash+
Unsplash+
