Rapid test for pandemics
Rapid tests have received a lot of attention during the Coronavirus pandemic, but as the number of tests available has increased, so has the confusion around the different types and what they can deliver. What if there were laboratory quality tests that could produce a result in just a few minutes?
Rapid tests have the potential to facilitate broader screening and have been discussed in many countries as a tool to lift coronavirus restrictions, however testing capacities as well as the variability of the tests themselves have limited their implementation.
The most common, safest method for diagnosing COVD-19 infection is PCR (Polymerase Chain Reaction). In PCR, the genetic material of the virus is detected in the laboratory to assess viral infection. Typically, PCR test results are available after several hours, but it often takes much longer for the patient to receive their result depending on infrastructure. This is because it takes time for a sample to make its way to the laboratory where the evaluation is made by expert laboratory personnel. Once that evaluation is available, results then need to go back to the test site, and finally to the patient.During this pandemic, PCR-based rapid tests have become available, but with some limitations: they are typically the most expensive option, which limits their suitability for screening. Furthermore, the definition of rapid when it comes to PCR tests can vary wildly, from 15 minutes (e.g. ID Now) to several hours (e.g. Vivalytic).
Immune response vs viraemia
The vast majority of rapid tests to become available in the recent months have been antibody tests. These tests detect a person’s antibody immune response to the Sars-CoV-2 virus rather than the virus itself. Therefore, these tests are not suitable for detecting acute infection with Sars-CoV-2 virus. Furthermore, it remains unclear whether the antibodies produced as a response to Sars-CoV-2 infection are protective for future infection and if so, for how long.
Finally, there are antigen-based rapid tests, which directly detect components of the Sars-CoV-2 virus itself in approximately 20 minutes. These tests, while less expensive and faster than PCR, have been limited in how sensitively they can detect infection. Relative to PCR, current antigen-based tests are about a thousand times less sensitive. This means that while they may be faster, less expensive, and easier to implement, they have a much higher potential to produce large numbers of false-negative results, missing many active infections.
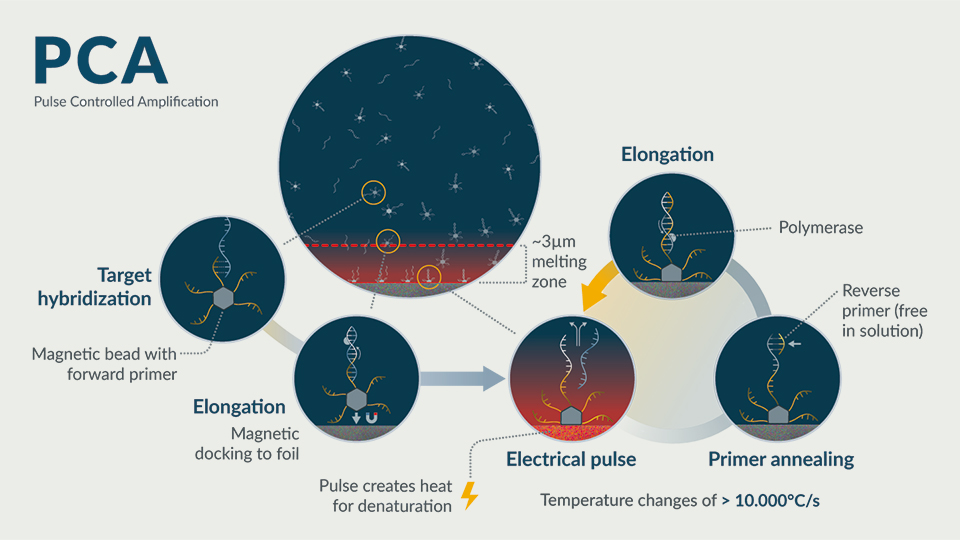
Hybrid PCA tests
New technologies that can deliver the best of PCR tests (high sensitivity) as well as antigen-based tests (speed and ease of use) have been lacking. One new technology that can deliver across these parameters is Pulse Controlled Amplification (PCA), a next-generation PCR technology developed by GNA Biosolutions in Germany. PCA technology functions similarly to PCR at the chemical level, utilising conventional polymerases and primers, with the needed sensitivity, but with tests that take minutes rather than hours (Figure 1).
Portable, energy efficient and even disposable PCA-based systems are currently in development for Sars-CoV-2 testing and promise to deliver new powerful solutions for combatting Covid-19 and other infectious disease threats. With this new generation of molecular diagnostic systems as well as better integration of test reporting and result tracking, a safe test for coronavirus that can be purchased in a pharmacy, or even performed at home, is becoming a possibility within reach.
This article was published in the European Biotechnology News Magazine Summer Edition 2020.



 Photo: Carolina Garcia Tavizon, via Unsplash
Photo: Carolina Garcia Tavizon, via Unsplash  ©FabienMalot
©FabienMalot