PulseSight to disrupt AMD space
PulseSight Therapeutics SAS was launched on Wednesday, backed by seed financing from Pureos Bioventures and ND Capital. The company introduces a disruptive delivery platform in ophthalmology biotech, focusing on the development of non-viral gene therapies coupled with minimally-invasive delivery technology.
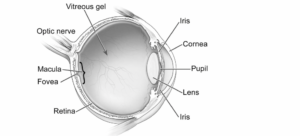
Aiming to address the pressing challenges of age-related macular diseases (AMD), including wet and dry AMD, along with geographic atrophy (GA), PulseSight aims to pioneer treatments that combat vision loss, particularly prevalent among the elderly population. At the core of PulseSight’s innovation lies its proprietary electro-transfection system, a delivery mechanism designed to precisely administer therapeutic DNA plasmids into the ciliary muscle, effectively targeting major eye diseases with high accuracy. Having already undergone validation in Phase I/II clinical studies, PulseSight’s platform showcases promising safety profiles and enduring clinical benefits.
With a focus on wet AMD, a disease area fraught with therapeutic challenges, PulseSight’s lead programme, PST-809, emerges as a potential paradigm shift. Combining a dual-gene plasmid encoding aflibercept and decorin, PST-809 not only targets vascular endothelial growth factor (VEGF) but also addresses angiogenesis and fibrosis, presenting a multifaceted approach to disease management. Preclinical studies have demonstrated PST-809’s superiority over intravitreal aflibercept in reducing vascular leakage and promoting retinal pigment epithelial (RPE) wound healing, thus holding promise for disease regression and sustained vision preservation. Moreover, PST-809 offers the prospect of enhancing patient compliance by significantly reducing the frequency of anti-VEGF injections to once every six months, thereby alleviating the burden of treatment for individuals battling this chronic disease.
The second programme, PST-611 targeting geographic atrophy (GA) in late-stage dry AMD, utilises the same delivery technology. This approach involves a plasmid encoding the human transferrin protein, a natural iron transporter crucial for regulating iron levels in the eye. PST-611 shows promise in addressing the complex pathophysiological pathways associated with GA/dry AMD. Preclinical studies across various disease models demonstrate the favourable effects of transferrin, including iron removal, reduction of oxidative stress, preservation of retinal pigment epithelium integrity, and prevention of retinal degeneration and vision loss. Beyond GA, PST-611 holds potential for treating other neurodegenerative retinal disorders such as glaucoma or retinitis pigmentosa. It has been granted orphan drug designation by the FDA and EMA, underscoring its therapeutic promise in these areas.
PulseSight Therapeutics SAS has secured seed investment from prominent venture capital investors Pureos Bioventures and ND Capital. Dominik Escher, founding partner of Pureos Bioventures, and Kostas Kaloulis, Venture Partner at ND Capital, have joined the company’s board. Pureos is delighted to partner with ND Capital to launch PulseSight, a company with technology and programs that has the potential to be truly life-changing for patients with diseases leading to sight loss, said Escher.
Judith Greciet, erstwhile CEO of Onxeo and President of Eisai France, will lead the company as CEO. Greciet commented: Our proprietary non-viral gene therapy approach provides unique features in terms of efficacy, safety and durability of effect and I am thrilled to have the opportunity to work with PulseSight Therapeutics’ highly experienced team and board to build the company’s future.