
Lilly‘s retatrutide delivers record weight loss
Lilly’s triple weight loss combination therapy retatrutide has shown 28.7% weight loss in Phase 3, but higher discontinuations vs. tirzepatide and semaglutide.
Eli Lilly’s retatrutide has exceeded top-end expectations in the Phase III TRIUMPH-4 trial, linking the triple agonist to 28.7% average weight loss among participants who completed 68 weeks of treatment. The results reinforce retatrutide’s potential to set a new benchmark in obesity and metabolic care. However, the readout was tempered by higher discontinuation rates compared with earlier studies.
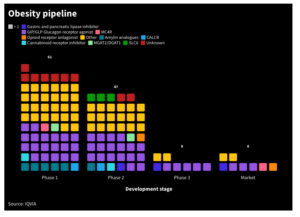
Retatrutide activates three metabolic peptide hormone receptors – GLP-1, GIP, and glucagon – expanding on the dual-agonist mechanism used in Lilly’s marketed drug tirzepatide (GLP-1 + GIP). TRIUMPH-4 enrolled 445 adults with obesity or overweight and compared once-weekly 9-mg and 12-mg doses against placebo.
Patients who completed the 68-week trial lost an average of 28.7% of baseline body weight on 12 mg – equivalent to 32.3 kg from a starting weight of 112.7 kg. Those on 9 mg lost 26.4%, or 29.1 kg. When all randomized patients were included, including those who stopped treatment early, the 12-mg arm averaged 23.7% weight loss at Week 68.
Analysts at BMO Capital Markets had set a 25% weight-loss bull case, which the Phase 3 results exceeded. TRIUMPH-4 also outperformed the earlier Phase 2 trial, which reported up to 24.2% weight loss at Week 48.
Tolerability remained the principal concern.
Adverse-event-related discontinuations reached 18.2% in the 12-mg group and 12.2% in the 9-mg group, versus 4% in the placebo arm. Phase 2 discontinuations reached up to 16%, indicating that the tolerability profile is consistent but significant. An uncomfortable skin sensation, dysesthesia, occurred in around 20% of patients on 12 mg, usually mild and rarely leading to withdrawal.
Lilly said discontinuation rates were highly correlated with baseline BMI, noting that some patients discontinued due to perceived excessive weight loss. Restricting the analysis to participants with BMI ≥35 reduced discontinuations to 8.8% (9 mg) and 12.1% (12 mg), versus 4.8% for placebo.
Despite beating the efficacy bull case, the discontinuation signal limited the market reaction. Lilly shares rose 1% in premarket trading—modest movement for a near-trillion-dollar company.
Market context and outlook
Retatrutide enters a rapidly expanding obesity-drug landscape defined by incretin-based therapies. Tirzepatide has demonstrated 22.5% weight loss at 72 weeks, while semaglutide typically shows ~15–17% reductions. With higher efficacy but more complex tolerability, retatrutide may appeal most to patients needing deeper weight loss or additional metabolic benefits.
The global obesity-drug market is expected to surpass US$100bn by the early 2030s. Retatrutide’s performance positions it as a potential category-shaping therapy, but real-world adherence and side-effect management will be essential to its long-term commercial impact.
Lilly plans seven additional Phase III readouts in 2025, including studies evaluating a 4-mg maintenance dose, which may offer a different balance of efficacy and tolerability.


 IQVIA
IQVIA
 MitoRx Therapeutics Ltd
MitoRx Therapeutics Ltd