
Topical DHT blockade demonstrates significant efficacy
Clascoterone 5% topical solution has demonstrated significant hair growth in Phase III trials, showing strong efficacy and a favourable safety profile in men with androgenetic alopecia.
Cosmo Pharmaceuticals NV, a global pharmaceutical company specialising in dermatological therapies and innovative treatments, has reported the results of its two pivotal Phase III trials, SCALP‑1 (NCT05910450) and SCALP‑2 (NCT05914805), evaluating Clascoterone 5% topical solution. In total, 1,465 male patients participated, representing the largest Phase III programme to date for a topical treatment of androgenetic alopecia (AGA).
The primary endpoint was the change in Target-Area Hair Count (TAHC) within defined scalp regions. SCALP‑1 demonstrated a relative improvement versus placebo of 539%, while SCALP‑2 showed 168%. Patient reported outcomes (PROs) supported these findings, with the combined analysis indicating statistically significant improvements in perceived hair growth. The safety profile was favourable, with treatment-emergent adverse events comparable to placebo and no systemic effects observed.
These trials confirm that Clascoterone locally blocks DHT at the hair follicle receptor, halts miniaturisation, and can reactivate vellus hairs without systemic hormonal exposure. Limitations remain: areas without active follicles or with long-standing baldness show no response. DHT-targeted therapies stabilise and partially regenerate existing follicles but cannot create entirely new hair follicles.
In parallel, Mallia Innovations, a biopharmaceutical company focusing on regenerative dermatology, is developing a sCD83-based candidate for cosmetic and pharmaceutical use. Preclinical models indicate activation of follicular stem cell niches, protection against follicular apoptosis, and hair neogenesis. Clinical data are pending; this regenerative approach may target scalp regions inaccessible to DHT-blocking therapies, representing a potential paradigm shift in AGA treatment.


![mode-of-action details[73] Mode of action of sCD-83](https://european-biotechnology.com/wp-content/uploads/2025/08/mode-of-action-details73-300x210.png) Mallia
Mallia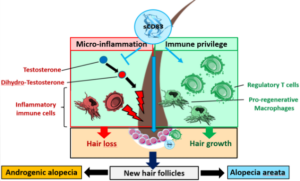 Mallia Therapeutics
Mallia Therapeutics