Siemens Healthineers enters CRISPR diagnostics space
Siemens Healthineers have participated in a US$25m Series A extension of the CRISPR diagnostics specialist VedaBio Inc and signed a strategic agreement.
In an initial Series A round, the US-based VedBio Inc raised US$40m, led by OMX Ventures, which also acted as lead investor in the Series A extension. The funding includes a strategic agreement with Siemens Healthineers to further develop and globally distribute the US company’s CRISPR Cascade™ diagnostics platform.
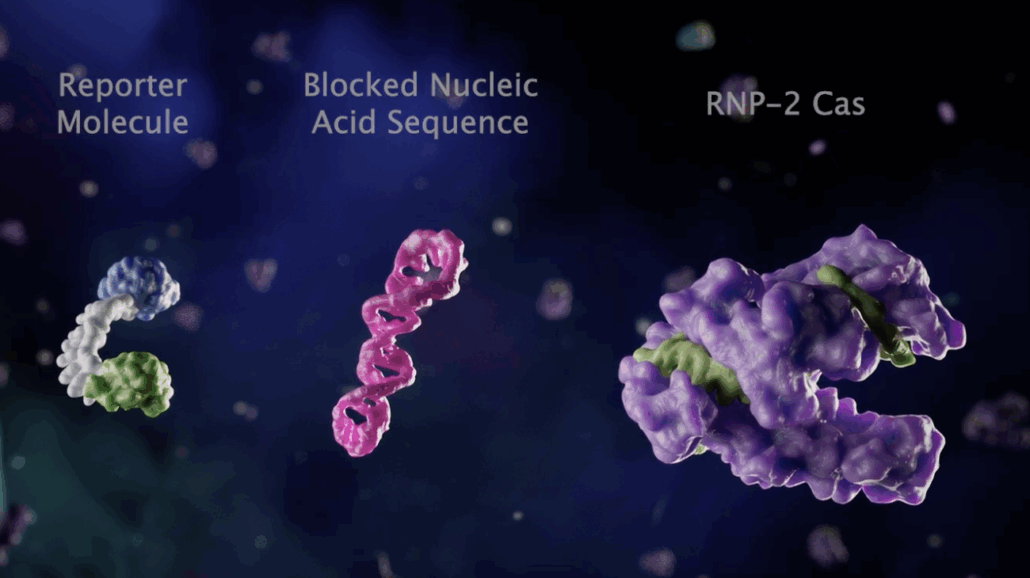
Conventional CRISPR diagnostic platforms such as SHERLOCK or DETECTR utilise Cas enzymes like Cas12 or Cas13, which, after binding the guide strand, trigger secondary, non-specific nuclease activity. This activity can then cleave a fluorescent reporter or other signal molecule, thereby detecting the presence of the target sequence.
In contrast to most molecular diagnostic tests (PCR, LAMP, RPA), which require prior nucleic acid amplification to generate sufficient material for detection, VedaBio’s method can detect extremely low amounts of target nucleic acids without amplification, using signal amplification. This saves time and reduces amplification errors. Because amplification steps are not required, the test can be performed within 10 to 30 minutes in clinical or point-of-care settings. The collaboration with Siemens Healthineers targets the clinical laboratory diagnostics market and integration into automated lab workflows. An earlier agreement with Mammoth Biosciences focuses on optimising the amplification-free method.
VedaBio’s amplification-free, CRISPR-based approach provides an innovative solution to address significant gaps in patient care.
This announcement follows VedBio’s recently disclosed non-exclusive license agreement with Mammoth Biosciences Inc, which expanded the company’s access to key CRISPR technologies. Together, these milestones underscore VedaBio’s momentum in building a next-generation molecular diagnostics platform while continuing to advance its product development.


 EIB, Oscar Romano
EIB, Oscar Romano adobe stock photos - Miha Creative
adobe stock photos - Miha Creative  Pleiotrope - https://commons.wikimedia.org/w/index.php?curid=15999847
Pleiotrope - https://commons.wikimedia.org/w/index.php?curid=15999847