
Roche moves obesity asset CT-388 into Phase III
Swiss Roche AG will advance its injectable obesity lead CT-388 into Phase III studies starting in H1/2026. With combination therapies, the Swiss pharma major aims at becoming obesity company No. 3 by 2030, Roche Pharma CEO Teresa Graham said at Roche’s Pharma Day in London.
“Our goal is to become a top 3 player in the obesity drug market, and I want you to know I’m serious about this goal,” Graham said, Reuters reported. During her presentation, Graham noted that the company has six assets in the pipeline for obesity and related conditions such as type 2 diabetes, adding they could all hit the market by 2030, with some potentially becoming blockbusters.
In March, Roche signed a licensing deal with Zealand Pharma A/S, which has also partnered with Boehringer Ingelheim in obesity, to co-develop and co-commercialize petrelintide, an amylin analog, as a standalone therapy and a fixed-dose combination with CT-388. The Swiss pharma acquired CT-388 through its US$2.7bn acquisition of Carmot Therapeutics in December 2023. In Phase I trials, the subcutaneously administered dual GLP-1/GIP receptor agonist achieved a placebo-adjusted weight loss of 18.8% at 24 weeks, outperforming Novo Nordisk’s GLP-1 blockbuster Wegovy, which achieved 14.9% weight loss by 68 weeks, but not Eli Lilly’s GLP-1/GIP-RA Zepbound (tirzepatide), which reduces weight by up to 22.5% at the 15 mg dose after 72 weeks in the SURMOUNT-1 Phase III study. According to Graham, combination with the MASH candidate FGF21 mimic pegozafermin, which Roche acquired through the recent takeover of 89bio Inc, will provide another angle to address obesity comorbidities.
The news came after Pfizer announced the acquisition of Metsera Inc. for US$4.9bn. The takeover will add four incretin and amylin programs to Pfizer’s pipeline, including MET-097i, a weekly and monthly injectable GLP-1 RA in Phase II development; MET-233i, a monthly amylin analog candidate being evaluated as monotherapy and in combination with MET-097i in Phase I development; two oral GLP-1 RA candidates expected to begin clinical trials imminently; and additional preclinical nutrient-stimulated hormone therapeutics.
Incretin-free approaches that do not reduce muscle mass through reprogramming white fat cells into energy consuming brown adipocytes, reported by a Danish/German reseach team and Italian Resalis Therapeutics srl, which partnered with Sanofi, are still early stage.



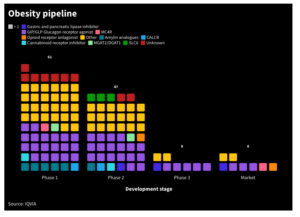 IQVIA
IQVIA