Dewpoint Therapeutics and Mitsubishi Tanabe partner on ALS therapy
Dewpoint Therapeutics Inc and Mitsubishi Tanabe Pharma Corporation have formed a collaboration, valued at up to $480m, to develop a novel small molecule condensate modulator for ALS, leveraging groundbreaking European research in condensate biology.
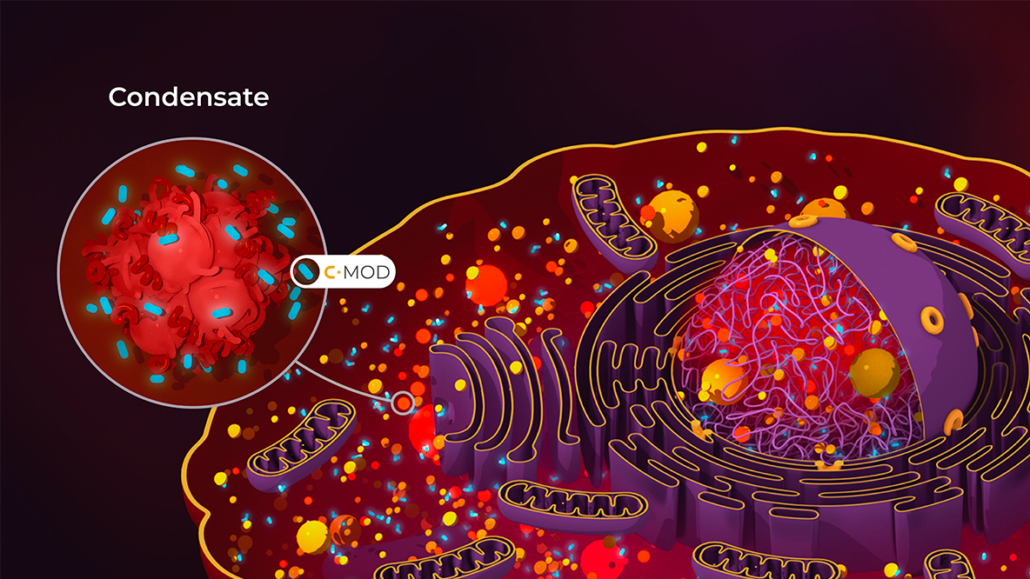
Japanese Mitsubishi Tanabe has licenced Dewpoint Therapeutics Inc’s condensate biology platform to expand its drug pipeline in amyotrophic lateral sclerosis (ALS). The R&D collaboration builds on Dewpoint’s work in condensate biology, a field originating from discoveries at the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden (MPI-CBG). Dewpoint’s c-mod technology aims to address the mislocalisation of TAR DNA-binding protein 43 (TDP-43), a key factor in over 97% of ALS cases. By restoring TDP-43’s correct localisation and function, this approach has potential applications in ALS and other neurodegenerative conditions.
“We are delighted to collaborate with MTPC in advancing our condensate-modulating approach to address the critical unmet need in the treatment of ALS,” said Ameet Nathwani, CEO of Dewpoint Therapeutics. “Our c-mod was discovered and developed through Dewpoint’s pioneering condensate-targeted platform.”
European roots
Dewpoint’s technological foundation stems from discoveries in Dresden and Boston. Co-founded by Anthony Hyman of the Max Planck Institute (MPI-CBG) and Richard Young of the Whitehead Institute, Dewpoint has been exploring biomolecular condensates—membraneless organelle-like biochemical submileus that regulate cellular processes. This approach has drawn significant investor interest, including a US$60m Series A round led by Polaris Partners and Bayer’s venture arm, Leaps by Bayer and a US$77m Series B financing.
Dr. Ivan Baines, COO of the MPI-CBG, noted, “Phase transitions and the role of biological condensates represent a paradigm shift as well as a new field in biology. We are thrilled to see research findings from MPI-CBG as center stage of the new field of phase transitions and to be working with Dewpoint Therapeutics.”
Strategic collaboration with Mitsubishi Tanabe
Mitsubishi Tanabe, one major pharma player in ALS and the developer of edaravone, one of the few FDA-approved treatments for the condition, brings substantial expertise to the partnership. “By joining forces with Dewpoint, we aim to deliver meaningful innovations to patients and families affected by this devastating condition,” said Masao Nawano, Vice President and Head of Research Division at MTPC.
Under the agreement, Dewpoint will receive upfront payments and milestone-based funding, with MTPC holding an option for global licensing and commercialisation. Dewpoint will also earn royalties on net sales, should the therapy succeed.
Broadening the Applications of Condensate Biology
Dewpoint’s collaboration with MTPC highlights its broader strategy to utilise condensate biology across multiple therapeutic areas, including oncology, cardiopulmonary, and metabolic diseases. By leveraging its scientific platform, Dewpoint aims to address complex diseases and deliver novel treatments to patients worldwide.


 Rosa Therapeutics Inc
Rosa Therapeutics Inc adobe stock photos - deemerwha
adobe stock photos - deemerwha Bristol Myers Squibb
Bristol Myers Squibb