Sensorion achieves milestones in Phase II trial
French Sensorion SA has reported new efficacy endpoints data from SENS-401 in a Phase II study for the preservation of residual hearing loss.
Following cochlear implantation, SENS-401, a 5HT3R antagonist that blocks the CalN pathway, reduced sudden hearing loss as measured by the change of hearing threshold from baseline to the end of the treatment period in the implanted ear at several frequencies. Study entry criteria required patients to have a pure tone audiometry (PTA) threshold of 80 dB or better at 500 Hz, defined as indicating a minimal level of residual hearing.
Six weeks post cochlear implantation, the mean hearing loss at 500 Hz was 19 dB for patients treated with SENS-401 (N=16) compared to 32 dB for the control group (N=8). A clinically meaningful difference was also observed for the mean of 250, 500 and 750 Hz with 16 dB in the SENS-401-treated group compared to 31 dB in the control group. These results remained until the last study visit fourteen weeks after cochlear implantation and confirm the key role of SENS-401 in preserving residual hearing.
Back in March, Sensorion reported that it had successfully met the primary endpoint of the SENS-401 study for the preservation of residual hearing in adult patients following cochlear implantation. The presence of SENS-401 in the perilymph at a level compatible with potential therapeutic efficacy was confirmed in 100% of the patients sampled, seven days after the start of the treatment. These results confirm that SENS-401 administered orally crosses the labyrinth barrier. The study is developed in collaboration with Cochlear Ltd, a global leader in implantable hearing solutions. According to Nawal Ouzren, Sensorion’s Chief Executive Officer, full final data later will be presented in Q3 2024.
SENS-401 (Arazasetron)is an orally available small molecule designed to protect and preserve inner ear tissue from damage responsible of progressive or sequelae hearing impairment. Sensorion currently develops SENS-401 in a Phase 2a for the prevention of residual hearing loss in patients scheduled for cochlear implantation and in a Phase 2 clinical trial for the prevention of Cisplatin-Induced Ototoxicity. SENS-401 has been granted Orphan Drug Designation by the EMA in Europe for the treatment of sudden sensorineural hearing loss, and by the FDA in the U.S. for the prevention of platinum-induced ototoxicity in pediatric population.


 eXmoor Pharma
eXmoor Pharma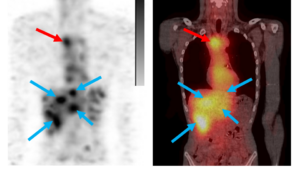 Molecular Partners AG & NuMeRI, presentation Feb. 2026
Molecular Partners AG & NuMeRI, presentation Feb. 2026