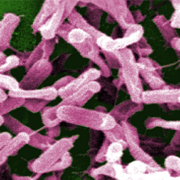
The decision of the vaccine arm of French drug giant Sanofi was based on an independent interim analysis of a pivotal Phase III study enroling up to 16,500 people at risk of Clostridium difficile infection (CDI) for vaccination with the jab that has FDA Fast Track designation.
ADVERTISEMENT
OPIS Accelerated approval for orphan drugs and the possibility to have market authorisation
after a successful Phase II trial have made research in rare diseases more attractive to sponsors.
However, challenges and uncertainties remain numerous and designing scientifi cally robust,
patient-centered trials requires proper conceptualisation.
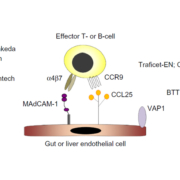
Shire has received FDA Orphan Drug Status for its anti-MAdCAM Antibody SHP647 to treat pediatric ulverative colitis.