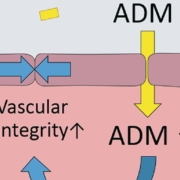
German Adrenomed AG has decided to start clinical Phase II tests of the very first personalised treatment for the six million patients with acute congestive heart failure. Large trials suggest that the company can stratify patients resistant to diuretics standard therapy by a proprietary companion diagnostics blood test. Adrenomed will assess whether its first-in-class antibody adrecizumab can reverse the congestion that that manifests as pulmonary edema.
ADVERTISEMENT
The French biotech said that its lead therapeutic candidate demonstrated the first reduction in HIV reservoirs ever observed in chronically infected HIV patients. Following the news, the company’s share price more than doubled.