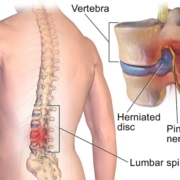
30.08.2016 – Swiss biopharma company Ferring has acquired the exclusive development and commercialisation rights to Seikagaku’s Phase III chemonucleolytic leg pain treatment for €85m.
ADVERTISEMENT
Brussels-based vaccine developer Univac has appointed a new CEO. In March, Stefano Malvolti joined Univac from Gavi, the Vaccine Alliance, where he was responsible for rolling out the vaccine development programmes of the public-private partnership.
Adocia said it would discontinue its diabetic foot ulcer programme after its lead product BioChaperone PDGF disappointed in Phase III. The French company will turn the focus on its earlier-stage products.
24.08.2016 – Pharma major Pfizer is putting the cherry on top of its multi-billion-dollar takeover of Medivation with the acquisition of AstraZeneca’s antibiotics for a cool US$1.5bn. With the divestment, AZ aims to further sharpen its focus.
23.08.2016 – Cambridge University-allied investor Cambridge Innovation Capital has raised £75m (€87m) to reinvest in technology and healthcare companies. Among the backers were Woodford Investment and Winton Ventures.
Faron Pharmaceuticals has someone new to oversee its drug development strategy. In early May, the Turku-based clinical stage biopharma company appointed Matti Karvonen as its new Medical Director.
FILTRODISC BIO SD is a filtration system which removes particles (e.g. cells, bacteria, yeast), impurities (e.g. HCP, DNA) and other turbid matter from process liquids (e.g. fermentation broths).
19.08.2016 – Together with partner Johnson & Johnson, Danish biotech Genmab is now one step closer to FDA approval with its combination treatment for multiple myeloma after its submission of a supplemental Biologics License Application. In July, Genmab was granted a Breakthrough Therapy Designation (BTD) for its blood cancer therapy.
Extrapolating the current growth in Germany, specialist search engine Viomedo could reach 100,000 monthly users in the beginning of 2017. Being fully operational for only half a year, this would be a sign for nothing less than – as Viomedo’s pharma partners would call it – a huge unmet need.
The European Medicines Agency’s new, accelerated route to approval for innovative drugs seems to be especially attractive for biotech companies. But Health Technology Assessment institutions warn that the lack of substantial data might interfere with reimbursement.