Cellectis in offering of US$175m ADS following asset swap
French allogenic CAR-T cell therapy developer Cellectis is set to launch an underwritten public offering of $175m of its American Depositary Shares (ADS), each representing one ordinary share of Cellectis.
Underwriters will have a 30-day over-allotment option to purchase up to 15% of the aggregate offering size. Goldman Sachs & Co. LLC and Citigroup will act as book-runners together with Barclays. Nomura and Oppenheimer & Co. and Ladenburg Thalmann will also participate.
The announcement followed an asset swap of Pfizer’s CAR-T therapy development portfolio into the newly founded company Allogene Therapeutics, in which Pfizer will hold a minority stake of 25%. Allogene was formed with Series A financing of US$300m from a consortium that includes TPG, Vida Ventures, BellCo Capital, the University of California Office of the Chief Investment Officer and Pfizer, as well as others. Under the asset contribution agreement, Allogene receives the global development and commercialisation rights to 16 preclinical CAR-T licenses that Pfizer licensed from Cellectis and Servier, and one clinical asset licensed from Servier, UCART19 in 2014. Pfizer still will have an 8% ownership stake in Cellectis through an equity deal signed in 2014.
Cellectis said it will remain eligible to receive clinical and commercial milestone payments of up to US$2.8bn (US$185m per target for 15 targets) and royalties in the high single digits on net sales of any product that will be commercialised by Allogene under the agreement. Allogene was co-founded and led by former executives of Kite Pharma, whose autologous CAR-T cell assets were acquired by Gilead Sciences, Inc. last year. Allogene’s management team will include former Kite CEO Arie Belldegrun, and David Chang, former Executive Vice President R&D and CMO of Kite.
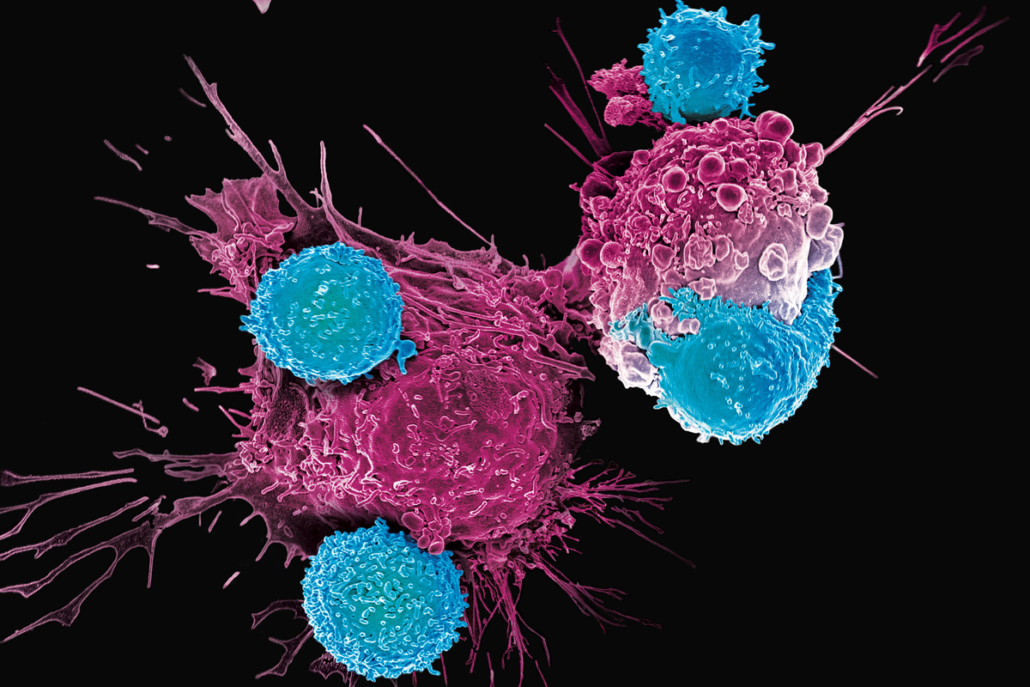
Development of Cellectis’ lead candidate UCART123 was delayed after clinical hold by the FDA following a death due to cytokine release syndrome. In November 2017, the FDA lifted the clinical hold after Cellectis proposed to change dosing. Cellectis’ CAR T platform use engineered T-cells from a healthy donor for use in multiple patients. This is distinct from autologous approaches that use a patient’s own T-cells to target tumor cells. Since manufacturing of autologous CAR T cell therapies is costly and complex, production of allogenic CAR-T cell therapies is expected to significantly reduce cost thus increasing profits for developers. According to Cellectis, UCARTs (Universal Chimeric Antigen Receptor T-cells) are off-the-shelf products that have the potential to be industrialized and standardised, with consistent pharmaceutical release criteria, over time and from batch to batch.


 SLAS - Alexandra Csuport Photography
SLAS - Alexandra Csuport Photography Phabioc GmbH
Phabioc GmbH