British drug developer Mission Therapeutics plc earns $20m milestone
British drug developer Mission Therapeutics plc has bagged a $20m milestone payment from Abbvie within the companies' neurodegenerative disease collaboration.
In the second major milestone of the companies’ research and preclinical development collaboration, AbbVie has nominated two deubiquitylating enzymes (DUB) targets to progress to the next stage of drug discovery. This selection follows supportive data from in vitro and in vivo Alzheimer’s and Parkinson’s disease models. As a result of the nomination, Mission will receive a milestone payment of $20 million from AbbVie.
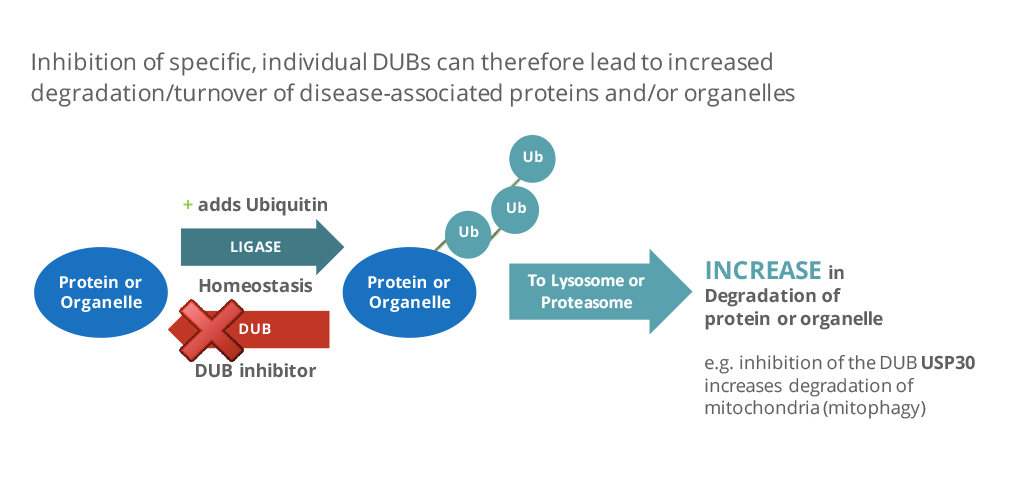
DUBs are increasingly being considered as attractive therapeutic targets due to their relevance to many diseases such as cancer, inflammation, neurodegeneration, muscle wasting and infectious disease.
Alzheimer’s and Parkinson’s diseases are the most common neurological disorders worldwide, with over 50 million people living with dementia and Alzheimer’s disease, and 10 million more with Parkinson’s. While there are treatments to help reduce symptoms, there are no treatments available to help stop or reverse progression. Both Alzheimer’s and Parkinson’s are associated with the accumulation of misfolded, proteins in the brain, which are believed to cause impaired function and death of nerve cells.
DUBs can help to maintain cell health by regulating the degradation of these proteins by the proteasome. Under the terms of the agreement, first announced in 2018, Mission and AbbVie are collaborating to identify novel DUB targets and discover associated inhibitor compounds for neurodegenerative diseases. AbbVie has the option to gain exclusive rights to develop and commercialize inhibitors of selected DUB targets and will pay Mission success-based milestones as well as royalty payments for each commercialized product.


 Brainomix
Brainomix BioNTech SE
BioNTech SE