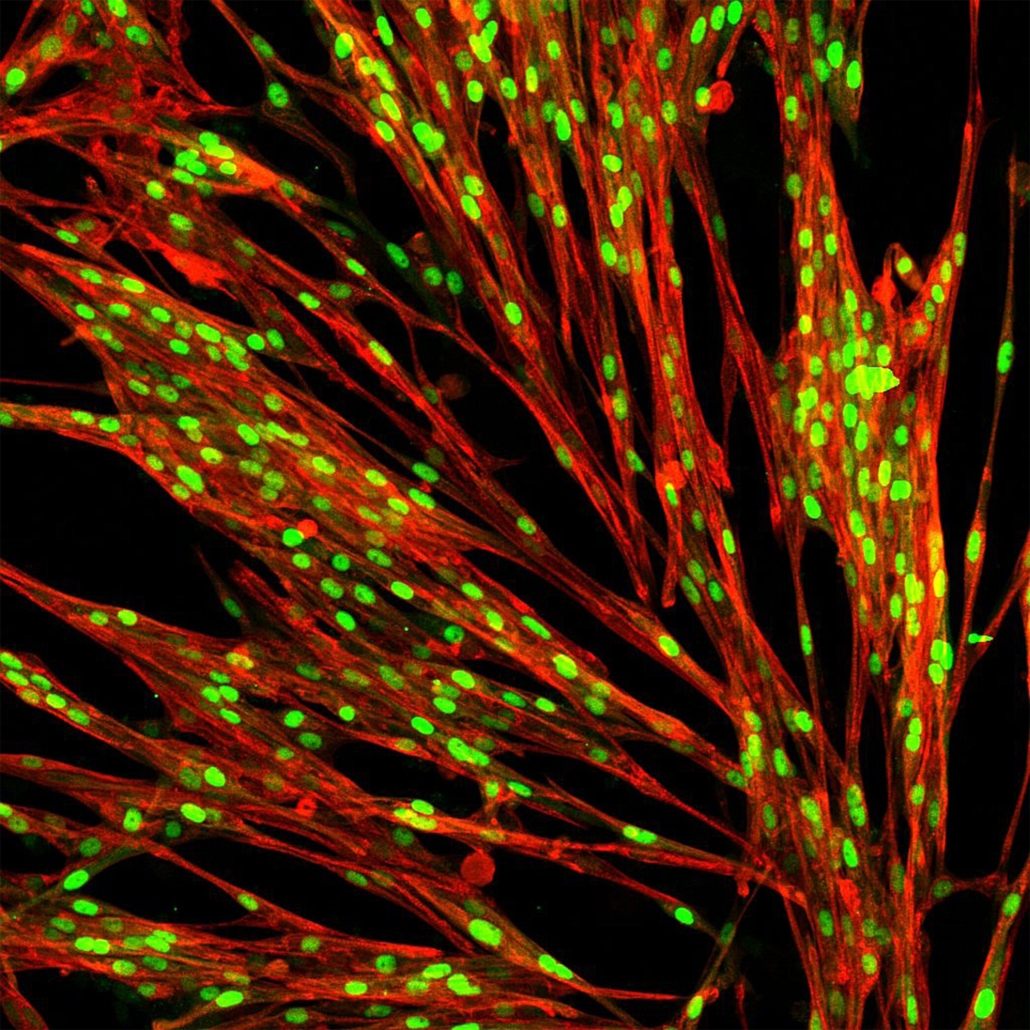
bit bio restarts with new management
Cell reprogramming specialist bit bio has expanded its management with renowned experts in the field.
Cambridge-based cell reprogramming specialist Elpis Biomed will be re-started under the new name bit bio with a new management and an extended scientific advisory board. Biotech entrepreneur and scientist Paul Morrill will support company founder and CEO Mark Kotter as Chief Business Officer; biotech veteran Florian Schuster has joined bit bio as CFO/COO. And cell reprogramming pioneer Marius Wernig, together with Ramy Ibrahim (MD) will help Chief Scientific Advisor Roger Pedersen to make the promise of merging stem cell biology with syntetic biology become reality.
For years, R&D in regenerative medicine, as well as drug and cell therapy development suffered from the lack of easy-to-use, standardised and scalable protocolls to differentiate and expand cell lines from induced pluripotent stem cells with more or less reproducible characteristics. Two IMI projects; Stembancc and EBISC, provided production and differentiation protocols to satisfy Big Pharma’s hunger for human cell models that cirumvent the limitations of animal testing. However they did not solve the major problem of direct cell programming: to reprogram a cell you must first silence its active transcription factors and then activate another set of transcription factors.
Founded by stem cell biologist and neurosurgeon Mark Kotter, Elpis Biomed, now bitbio , solved the problem by putting a gene switch and a new transcription factor programme into two safe harbour sites, which are privileged areas in the genome. As the switch is inducible by a drug, bit bio is able to activate a new set of transcription factors, which determine a specific cell’s items, on command.
bit bio calls its platform Opti-OX and says it’s designed for the efficient and consistent reprogramming of human cells for use in research, drug discovery, and cell therapy – the three markets the company is targeting. bit bio also says it is entering its next phase of development to transform drug discovery and enable a new generation of cell therapies. For R&D, bit bio already offers two products: skeletal myocytes and glutmatergic cortical neurons.
The next generation of medicine hinges on widespread access to human cells, said Kotter. That is the challenge we have set out to solve at bit bio.



 Unsplash+
Unsplash+