Novel RNA extraction method speeds up COVID-19 PCR tests
Although innovative biotechnologies are developed continuously, the method of nucleic acid extraction stayed unchanged for several decades. The classical bind-wash-elute approach seemed sufficient, but the COVID-19 pandemic uncovered the need for a new technique to be able to process multiple samples fast and in parallel. BioEcho developed a completely new technology, which simplifies and speeds up the process of RNA extraction dramatically.
The COVID-19 pandemic has hit the whole world with an unforeseeable impact, and it might not be the last viral pandemic we need to face. While it is extremely important to focus on vaccinations and treatments against SARS-COV2, we also need to prevent the virus from spreading. SARS-Cov2 PCR tests are the most reliable and easiest way to accomplish this. Clearly, this warning system is very time-sensitive, and several testing facilities were seeking new high-throughput solutions. The advantages of the patent-pending EchoLUTION technology are unmatched speed and easy integration into existing lab procedures combined with high sample throughput and purity. Currently around 12 % of all COVID-19 PCR tests in Germany and 50 % of all PCR tests in Austria already isolate the viral RNA with this technology.
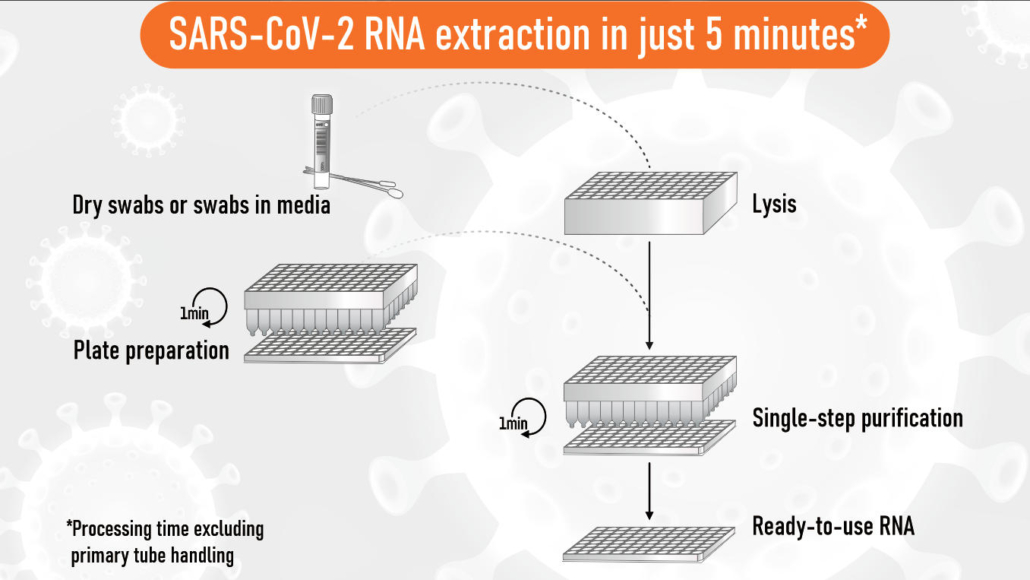
5-minute extraction technology
The innovative EchoLUTION Viral RNA/DNA Swab Kit is based on a novel lysis and purification technology using reverse chromatography. Nasopharyngeal viral swab solution is mixed with lysis buffer and directly transferred onto the purification plate for RNA extraction. The entire single-step purification process is completed within 5 minutes. Unlike conventional methods, all impurities are held back by the purification matrix and the RNA is running through untouched resulting in highly pure RNA since no inhibitory effects or reagent carry-over during the elution step are possible. This method is not only 20-fold faster compared to silica-based methods but at the same time the most sustainable approach: you save up to 70 % of plastic and packaging waste and significantly reduce the number of pipetting tips required.
COVID-19 testing
The extraction method works for any swab type (dry swabs, swabs in transport and inactivation media) and is compatible with commonly used SARS-Cov2 PCR assays. In combination with recently developed super-fast PCR (30 minutes) or LAMP assays (15 minutes), the EchoLUTION technology represents the fastest solution for COVID-19 testing available.
Future directions – Automation & CE-IVD
BioEcho is working with leading suppliers of high-throughput automation platforms to provide automated workflows for viral RNA isolation. Furthermore, the EchoLUTION Viral RNA/DNA Swab Kits will soon get CE-IVD certification to facilitate integration into the diagnostic laboratory routine. Contact BioEcho for a personalized virtual or on-site product demonstration or for further information on process automation options.
Contact
BioEcho Life Sciences GmbH
BioCampus Cologne
news@bioecho.de
Find out more on our website!
Tel. +49 (0)221-99 88 97-0



 Unsplash+
Unsplash+