A new treatment paradigm
Medigene is developing the next generation of effective TCR cell therapies aimed at establishing better control over cancer at reasonable production cost.
Medigene AG cultivates an in-house entrepreneurial start-up culture based on deep scientific expertise within the structures of an established biopharmaceutical company and follows a systematic discovery process in the T cell receptor (TCR) cell therapy space. With ongoing research to develop next-generation tools and to find important patient-individualised therapy targets for those with high medical need, Medigene is navigating toward its goals to transform cancer from an incurable disease to a controllable factor and to bring down production costs for highly innovative T cell therapies.
TCRs vs CAR-T cell therapy
CAR-T cells, because they use an antibody fragment to dock the highly active T cell onto the tumour cell, can only recognise surface proteins. A T cell receptor recognises small peptides that are brought to the surface by major histocompatibility complex (MHC) molecules from the intracellular space. Therefore, a TCR can look inside a cell and see what’s reflected. Many proteins get upregulated by a tumour, to give it growth and survival advantages. These proteins provide a differential window to target such molecules to be seen by TCRs.
TCR therapy works on the basis of a molecule that a T cell naturally uses. Auxiliary signaling pathways, which are coupled to that TCR, are all functioning independently and in parallel. Therefore, the approach should be safer, more regulated, and more powerful. Medigene expects to have better regulation and fewer side effects in the T cells using TCR-based approaches.
Cell therapy production
In cellular therapy, the product is a process. Establishment of a robust cell manufacturing process right from the very beginning of development is crucial, as it must deal with variations of patient-derived material but has to result in a product consistently delivering the expected therapeutic benefit. Medigene knows very much about immune cells: how they behave and how they can be manipulated to have particular characteristics. This helped tremendously in setting up the proprietary cell manufacturing process.
Medigene has established a GMP simulation lab onsite that can be used to explore new technologies out of the field of robotics and automation in the production process. Developing automation improvements have the potential to substantially reduce costs for cellular immunotherapies in the future.
Medigene is studying how to improve the characteristics of future cellular products. Potential side effects are still a concern with cellular immunotherapies, so new methods with improved control of T cell activity are needed. Medigene has developed an inducible T cell receptor that can be turned on and off in patients as needed.
First trial started
The trial of MDG1011 is the first such TCR trial in Germany. In order to gain regulatory approval, Medigene contributed to scientific advice meetings with the authorities to shape what to include with respect to the production process, and how the TCR-modified cells should be characterised, thereby setting the standards for future developments. From a patient and medical point of view, cell therapy is on its way to further revolutionise cancer treatment, becoming a standard treatment option, and turning cancer more into a chronic, if not even curable, disease.
Contact
Medigene AG
Business Development
bd@medigene.com
Tel.: +49-89-20 00 33-33 06



 eXmoor Pharma
eXmoor Pharma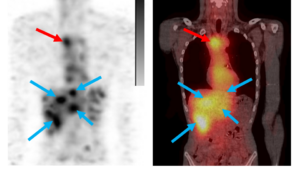 Molecular Partners AG & NuMeRI, presentation Feb. 2026
Molecular Partners AG & NuMeRI, presentation Feb. 2026