Zelluna Immunotherapy and Glycostem Therapeutics in licence deal
Zelluna Immunotherapy and Glycostem Therapeutics have partnered to develop allogeneic TCR-NK therapies.
TCR cell therapy specialist Zelluna Immunotherapy (Oslo) and NK cell manufacturing specialist Glycostem Therapeutics BV (Oss) have entered into a development, license and supply agreement in immuno-oncology. Zelluna did not publish any financial information concerning the deal, which aims at broadening the pipeline of the Norwegian cell therapy specialist.
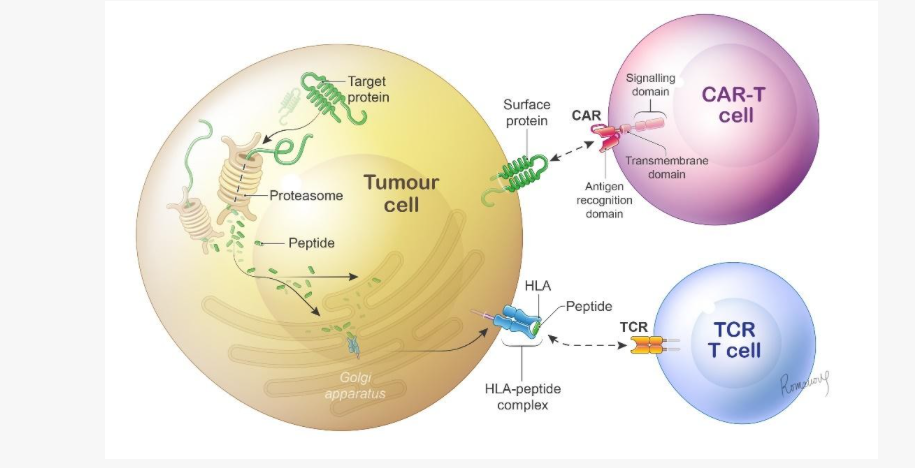
Under the agreement, Zelluna and Glycostem will combine their different expertises to develop and manufacture allogeneic TCR-guided NK-cell cancer therapies (TCR-NKs) targeting solid tumours. According to Zelluna, TCRs are able to target more tumour antigens than classical antibody-guided adoptive cell therapies. Under the deal, Glycostem will derive NK cells from donor-derived umbilical cord blood, develop and set up a GMP-compliant process to expand TCR-NKs and carry out clinical testing of allogeneic TCR-NK-cell product candidates. Zelluna, which already has a clinical pipeline of autologous TCR-T and allogeneic TCR-NK cell therapies, will lead the development and commercialisation of the TCR-NK products resulting from the collaboration.
In contrast to the currently hyped autologous NK cell therapies, manufacturing will not be an issue. Zelluna and Glycostem will be able to manufacture upfront a large number of patient doses to store and ship to clinical sites upon demand in an off-the-shelf manner. All current allogeneic cell therapy approaches, however, need immunosuppression because the recipients’ immune system attack foreign immune cells following cell transfer.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink