Urovant Sciences licenses OAB gene therapy
Urovant Sciences has licensed the global commercialisation rights for Ion Channel Innovations' gene therapy hMaxi-K for patients with overactive bladder (OAB) symptoms who have failed oral pharmacologic therapy.
hMaxi-K adds to the public Swiss company’s lead product candidate in OAB, vibegron, an oral, once-daily, small molecule beta-3 agonist, currently being tested in Phase III trials. hMaxi-K is a gene therapy initially developed for men with erectile dysfunction, https://www.ncbi.nlm.nih.gov/pubmed/17134370 ). It is a naked DNA plasmid carrying the human cDNA encoding hSlo (human slow-poke), the gene for the alpha, or pore-forming, subunit of the human smooth muscle Maxi-K channel. The increased expression of the maxi-K channel in corporal smooth muscle cells of men with ED normalizes smooth muscle tone regulatory mechanism. This is brought about by transient increased efflux of K+ across the cell membrane, hyperpolarization, and closure of voltage sensitive calcium channels with decreased influx of Ca2+ ions. There are no currently available FDA-approved gene therapy treatments for overactive bladder.
in OAB patients, hMaxi-K has been evaluated in two Phase I studies including a small, double-blind, placebo-controlled Phase Ib clinical trial as an intravesical injection in women with overactive bladder symptoms. Ion Channel Innovations found hMaxi-K to be generally well tolerated. Clinical results of a trial, on 13 patients indicated dose-dependent improvements in urinary urgency and frequency, achieving statistical significance (p<0.05) in the high dose cohort.
"We are pleased to add the gene therapy hMaxi-K to our clinical development portfolio. We are eager to study the potential of hMaxi-K as an alternative therapy for OAB patients who are not getting adequate relief from other therapies," said Keith A. Katkin, President and Chief Executive Officer of Urovant. "Urovant also has access to gene therapy expertise through the Roivant family of companies."
Urovant plans to meet with the FDA and initiate a Phase II clinical study in 2019 to investigate hMaxi-K as a novel treatment for OAB patients who have not responded to other pharmacological therapies.
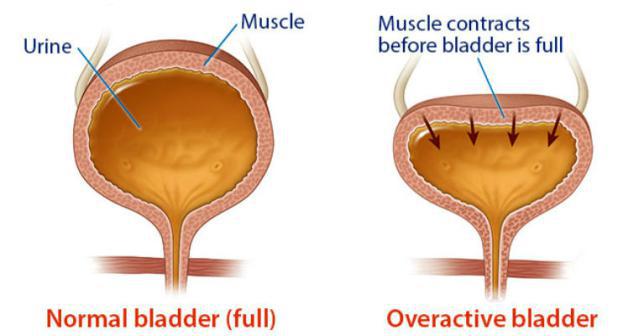
Overactive bladder is a clinical condition characterized by the sudden urge to urinate, with or without accidental urinary leakage, and usually with increased frequency that affects more than 30 million people over the age of 40 suffer in the US.


 Unsplash+
Unsplash+
 Bayer AG
Bayer AG