Takeda announced bid for Shire
Japanese drug giant Takeda Pharmaceutical Co. Ltd. has confirmed its intention to make a bid for orphan drug leader and neurology specialist Shire plc. The take-over offer has to be made until 25 April.
In a press release Shire said it has not received an official approach from Takeda.
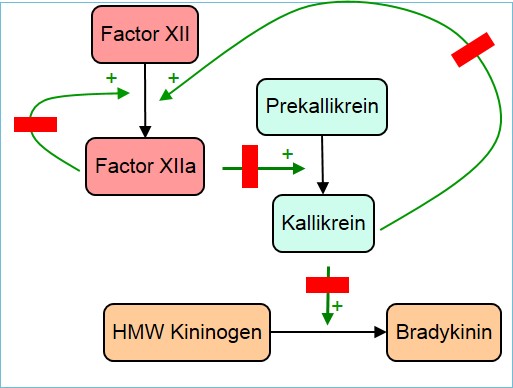
Following on the take-over rumours, Shire announced it achieved two important regulatory milestones for lanadelumab (DX-2930, originally developed by Dyax Corp.), a humanised therapeutic antibody to treat the rare disease hereditary angioedema (HAE) in patients 12 years and older. The European Medicines Agency confirmed that Shire’s data package for marketing authorisation application (MAA) is sufficient to begin an accelerated assessment of lanadelumab (SHP643). Additionally Health Canada accepted a New Drug Submission (NDS) under Priority Review for the subcutanously injected plasma kallikrein-binding antibody designed to prevent HAE attacks. In addition, lanadelumab has received priority review from the U.S. FDA and is expected to provide a decision by August 26, 2018, based on the Prescription Drug User Fee Act V action date
Shire announced, the rare, genetic disorder affects about 1 in 10,000 to 1 in 50,000 people worldwide. Andreas Busch, Ph.D., Executive Vice President, Head of Research and Development at Shire, said: Lanadelumab is the first monoclonal antibody under evaluation to prevent HAE attacks and has the potential to change the treatment paradigm for this rare disease, if approved.
The accelerate assessment of the EMA will reduce the time of evaluation from 210 to 150 days. Health Canada’s Priority Review will shorten the review timeline from 300 to 180 days. Filings are supported by data from four clinical trials. In a pivotal Phase III study enrolling 125 patients with type I/II HAE, Shire showed that subcutaneous administration of 300 mg lanadelumab once every two weeks resulted in an 87% reduction in the mean frequency of HAE attacks. The most common adverse event was injection site pain (29.3% placebo vs. 42.9 % combined lanadelumab arms).


 Alexander Sievert Photography
Alexander Sievert Photography  John Kiel - wikipedia
John Kiel - wikipedia Sanofi
Sanofi