SpliceBio lauches Phase I/II study on dual-AAV-based therapy for Stargardt Disease
Spanish SpliceBio has dosed the first patient with SB-007, the first dual AAV gene therapy in clinical development for Stargardt disease
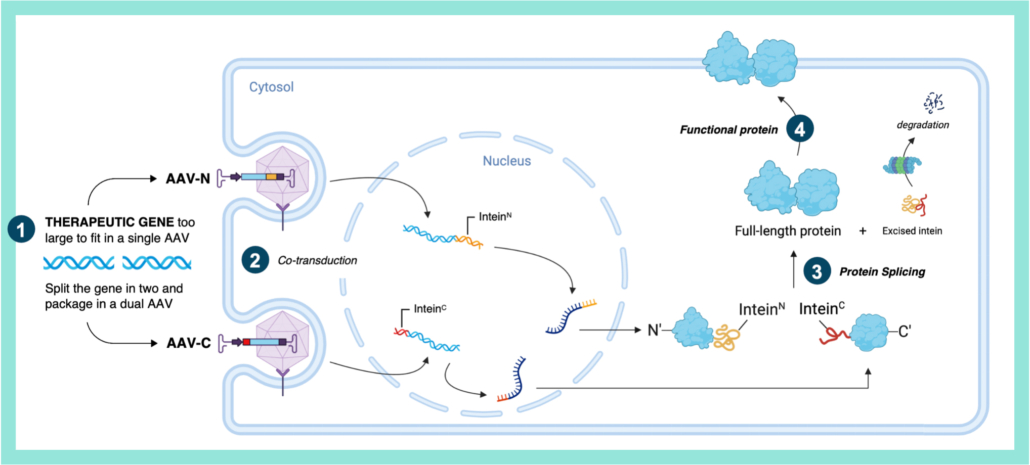
SpliceBio is using protein splicing to address rare familial disorders caused by mutations in large genes such as retinal disorder Stargardt disease. So far, Stargardt disease has remained elusive to gene therapies due to the large size of the underlying defective ABCA4 gene. However, using split inteins – auto-processing domains – to carry out protein splicing, it has become possible to repair very large genes through protein splicing. According to Vila-Perelló, Chief Executive Officer and co-founder of SpliceBio, “SB-007 is the first gene therapy in clinical development designed to restore expression of the full-length ABCA4 protein across all Stargardt disease patients, regardless of their mutations.”
Last December, the USFood & Drug Administration (FDA) cleared an investigational new drug (IND) application for SB-007. In the Phase I arm of the study, SpliceBio will evaluate the safety and efficacy of a single dose of SB-007 administered subretinally in patients with Stargardt disease.
In March 2024, SpliceBio launched the POLARIS trial, a pioneering company-sponsored natural history study of Stargardt disease designed to evaluate disease progression, refine endpoints, and streamline eligibility criteria for accelerated enrolment into the Phase 1/2 ASTRA study. This study will enable Stargardt disease patients to benefit from more precise diagnoses, more rigorous disease monitoring, and potentially faster access to innovative therapies.
An intein is an intervening protein domain that undergoes a unique posttranslational autoprocessing event, termed protein splicing. In this spontaneous process, the intein excises itself from the host protein and, in the process, ligates together the flanking N- and C-terminal residues (exteins) to form a native peptide bond. Tom Muir and colleagues, from which SpliceBio licenced its technology, however, found a way to eliminate sequence preferences at extein residues adjacent to the splice site using mutated split inteins for DNA-Polymerase III (DnaE).



 adobe stock photos - MikeCS Images
adobe stock photos - MikeCS Images