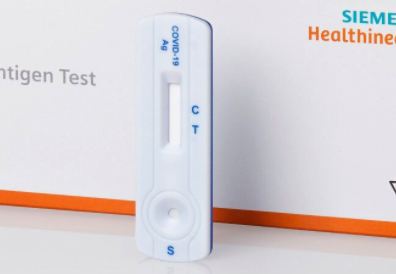
Siemens launches COVID-19 antigen lateral flow test
Siemens Healthineers has launched its Clinitest Rapid COVID-19 Antigen Test in Europe, which can identify persons infected with SARS-CoV-2 in 15 minutes.
As testing capacities for the SARS-CoV-2 gold standard RT-PCR are going to be exhausted upon the looming second wave of infections with the novel coronavirus, several companies have come up with antigen stripe testing as a quick alternative for the expensive and slow but accurate lab tests. Today, Siemens Healthineers has entered the race to getting a market share, particularly in the setting of rapid screening tests that detect SARS-CoV-2 within the first two weeks after infection.
Siemens Healthineers new offering is a point-of-care cassette test that does not require laboratory instruments or specialised lab personnel to administer, and it delivers results in 15 minutes. However as it works with nasopharyngeal swabs a physician is needed to make the swab. The licenced test shows a 96.7% sensitivity and 99.2% specificity based on a clinical study of 317 subjects. That does not significantly differ from the tests from competitors that have already received FDA emergency use authorisation such as Roche (sensitivity 96.5%; specificity 99.7%); Abbott Inc. (97.1% / 98.5%.); Lumira Dx Ltd (99.3% / 98.4%); Quidel Corp. (96.7% / 100%) but Becton Dickinson (84% /100%).
Innovative formats, such as the optical instant detection of viral proteins by means of functionalised carbon nanotubes, are currently still in the research stage, as well as CRISPR-dCas13a/Cas12-based tests from Nobel prizie winner’s Jennifer Doudna’s company Mammoth Biosicence, which provide a result after 5 minutes with comparable sensitivity to RT-PCR tests. Screening test are normally optimised for sensitivity and sort out the few false positive in subsequent confirmatory RT-PCR test.


 BioDlink
BioDlink
 Unsplash+
Unsplash+