Shire expands orphan drug portfolio
Shire has received FDA Orphan Drug Status for its anti-MAdCAM Antibody SHP647 to treat pediatric ulverative colitis.
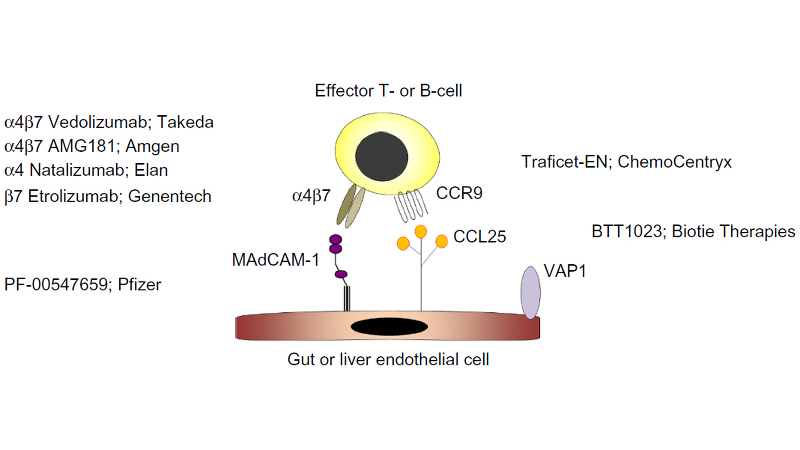
SHP647 (formerly known as PF-00547659), which Shire licenced from Pfizer in 2016, prevents lymphocyte extravasation into gut tissue by targeting the mucosal addressin cell adhesion molecule-1 (MAdCAM) on endothelial cells, which binds to the ?4?7 integrin at the surface of leukocytes. Inhibition of leukocyte translocation is believed to be a promising new approach to the management of Inflammatory Bowel Disease. The compound is currently in Phase III studies, for the treatment of moderately to severely active UC in adults. Pediatric study plans with SHP647 are under discussion with health authorities.
The orphan drug status will give Shire 7 years market exclusivity for SHP647. In the US, the reported incidence of ulcerative colitis in children aged 0-19 years is estimated between 0.34 and 2.9 per 100,000 children. Symptoms can be debilitating and include bloody diarrhea, tenesmus, abdominal pain, and in severe cases, weight loss, fatigue, and vomiting.
Debra Silberg, Therapeutic Area Development Lead for GI, Endocrine, and Metabolism at Shire, remarked, If approved, SHP647 holds the potential to help treat patients with ulcerative colitis. Shire’s study plans for SHP647 in the pediatric population align well with our commitment to address unmet patient need. Along with SHP647 Shire’s development pipeline includes currently 30 programmes.
According to figures of EvaluatePharma, Shire is currently the market leader in the orphan non-oncology field. The analysts predict Shire’s orphan drug revenues to grow from US$5.4bn in 2016 to US$9.8bn by 2022, cementing the company’s leadership in the non-oncology orphan drug market.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink