Roche’s Tecentriq prolongs OS in SCLC
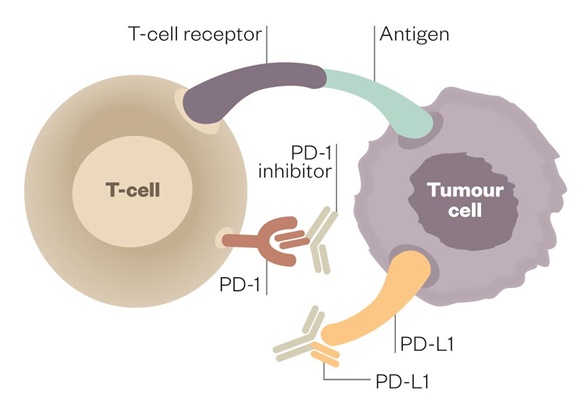
Cancer immunotherapy has been heralded as paradigm change in cancer treatment. Now, Roche's US arm Genentech announced that the company's anti-PD-L1 checkpoint inhibitor atezolizumab (Tecentriq) improved survival by only two months as first-line treatment for patients with the rare lung cancer SCLC.
According to results from the IMpower133 Phase III trail, presented at the world cancer conference on lung cancer (WCLC) and published in the New England Journal of Medicine, atezolizumab in combination with chemotherapy improved median overall survival and progression-free survival (the co-prinary endpoints) significantly over chemotherapy in patients with previously-untreated extensive-stage small cell lung cancer (ES-SCLC). Headline results have been previously reported by Genentech.
A total of 201 patients were randomly assigned to the atezolizumab group, and 202 patients to the placebo group. Following four induction cycles of 21 days on carboplatin and etoposide, patients either received atezolizumab + chemo or placebo + chemo. At a median follow-up of 13.9 months, the median overall survival was 12.3 months in the atezolizumab group and 10.3 months in the placebo group (hazard ratio for death, 0.70; 95% confidence interval [CI], 0.54 to 0.91; P=0.007). The median progression-free survival was 5.2 months and 4.3 months, respectively (hazard ratio for disease progression or death, 0.77; 95% CI, 0.62 to 0.96; P=0.02). The safety profile of atezolizumab plus carboplatin and etoposide was consistent with the previously reported safety profile of the individual agents, with no new findings observed.
The data will be submitted for approval in the US and other markets.
Two other checkpoint inhibitors in late-stage clinical development target the same patient population: Merck & Co’s PD-1 inhibitor pembrolizumab (Keytruda) and AstraZeneca’a PD-L1 inhibitor durvalumab (Imfinzi), which both are expected to finish clinical testing in Q1/2019. Interim overall survival data with durvalumab + chemoradiotherapy – also reported in NEJM – pointed to a 12-month overall survival rate of 83.1% (95% CI, 79.4 to 86.2) in the durvalumab group, as compared with 75.3% (95% CI, 69.2 to 80.4) in the placebo group. Roche reported a 1-year overall survival rate of 51.7% in the atezolizumab group and 38.2% in the placebo group. However, trial design and randomisation was different.
In August, the FDA granted accelerated approval to Bristol-Myers Squibb’s PD-1 inhibitor nivolumab (Opdivo) as second-line treatment for SCLC in patients who were retractive to platinum-based chemotherapy.
The success with immune checkpoint inhibitors most recently was challenged by reports that a fraction of patients with the lung cancer NSCLC (13.8%) and other cancers, who received one of the four approved PD1/PD-L1 blockers, developed hyperproliferative syndrome (HPS), with reduced OS and increase in metastatic lesions after 12 months follow-up compared to standard chemotherapy. Currently, there is no means to predict who will develop HPS. In the light of the high cost-per-patient (>$100,000) of cancer treatments with immune checkpoint modulators, biomarkers for stratification of responders to checkpoint inhibitor therapy and patients at risk to develop HPS are urgently needed.


 Bayer AG
Bayer AG