![mode-of-action details[73] Mode of action of sCD-83](https://european-biotechnology.com/wp-content/uploads/2025/08/mode-of-action-details73-1030x722.png)
Mallia Aesthetics is about to launch MAL-838
Germany’s Mallia Aesthetics GmbH has announced the achievement of key regulatory milestones in preparation for the market launch of its sCD83-based hair growth stimulant, MAL-838, later this year.
Mallia Aesthetics parent company, Mallia Innovations GmbH, is pursuing a novel approach to commercialising its sCD83-based compound for hair growth. Through its subsidiary Mallia Aesthetics GmbH, the holding company aims to bring the active ingredient MAL-838 to market under the EU Cosmetics Regulation before the end of the year. In parallel, Mallia Therapeutics is developing a separate formulation of sCD83 as a medicinal product for the treatment of androgenetic alopecia and alopecia areata.
Mallia Aesthetics GmbH reports having reached several major milestones in the development and regulatory validation of MAL-838— a hormone-free product line under the brand 8T3, which utilises the human soluble protein sCD83. Laboratory studies indicate that sCD83 stimulates hair growth, activates growth-related signalling pathways in human tissue samples, and induced the formation of new hair follicles in various model systems. However, under EU Cosmetics Regulation, the company is not permitted to promote these effects, as such claims are reserved for medicinal products.
Ahead of the planned fourth-quarter launch, Mallia Aesthetics has completed the safety assessment of all 8T3 products in accordance with EU Cosmetics Regulation 1223/2009. An independent laboratory confirmed the safety of MAL-838 through comprehensive in vitro and in silico testing. The resulting safety reports confirm that both MAL-838 and all 8T3 formulations can be used safely, thereby clearing the way for their marketing as cosmetics.
Concurrently, MAL-838 has been officially registered in the European Cosmetic Ingredients Database, the EU’s official register of cosmetic ingredients, confirming both regulatory compliance and market readiness. The compound will appear on product labelling under the INCI name sh-Polypeptide-167.
Independent testing has also certified the 8T3 formulations containing MAL-838 as microbiome-friendly. Unlike solvent-based hair growth agents, they do not disrupt the healthy skin microbiome.
The immunosuppressive soluble CD83 protein (sCD83), co-identified by Mallia co-founder Prof Dr Steinkasserer, is being developed for topical treatment of hair loss (MAL-856) and for the stimulation of hair growth (MAL-838). It activates regulatory T cells (Tregs), which interact with hair follicles, inhibits follicular cell death, and directly stimulates follicular stem cells, resulting in new hair growth. As a 45 kDa protein, sCD83 does not penetrate the skin and therefore does not act systemically.


 Office of Congresswoman Ayanna Pressley - https://pressley.house.gov/press-kit/
Office of Congresswoman Ayanna Pressley - https://pressley.house.gov/press-kit/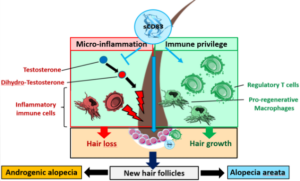 Mallia Therapeutics
Mallia Therapeutics