French Ipsen inks US$610m biobucks deal with Biomunex SA
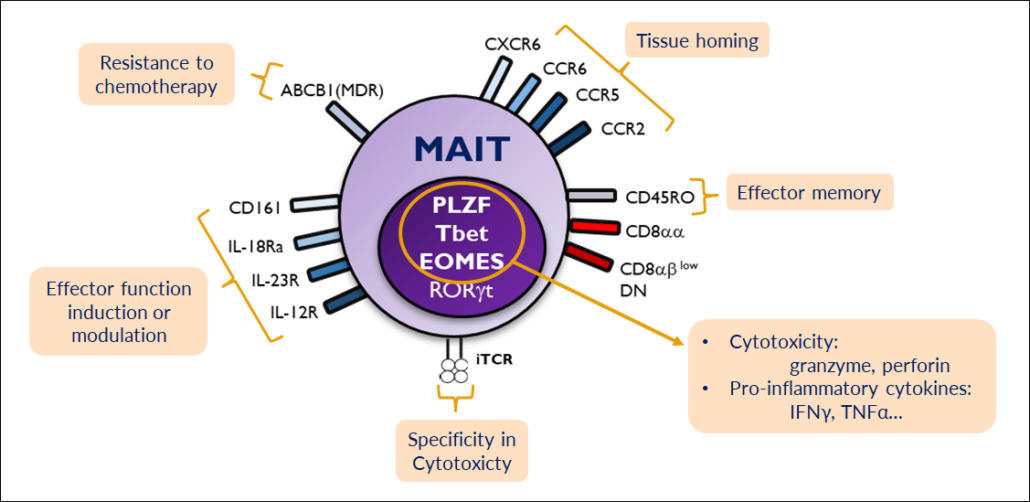
French Ipsen has exclusively licensed BMX-502, a T-cell engager (TCE) from Biommunex SA, which prevents common problems of TCEs such as cytokine release syndrome and T cell exhaustion by using a specific mucosal T cell subpopulation abundant in the human body.
Ipsen announced that it will pay US$610m for the commercialisation rights on Biomunex SA’s preclinical first-in-class MAIT (Mucosal Associated Invariant T cells) cell engager BMx-502, including success-dependent milestone payments. Under the terms of the agreement, Biomunex will complete the IND-enabling package. Ipsen will assume responsibility for Phase I preparation activities, including submission of the Investigational New Drug (IND) application, and all subsequent clinical-development and global commercialisation activities.
MAIT cell engagers are bispecific antibodies that link up a targeting antibody that is directed against a tumour-associated antigen (TAA) with an unconventional population of T cells that neither induce Treg nor CD4 activation, preventing immune exhaustion in solid tumours. BMX-502 targets GPC3, a TAA found on several solid tumours. Because MAIT cells, which represent about 20% of the entire T cell population in the body, do not carry a CD3 receptor, they do not induce cytokine relase syndrome (CRS) but just trigger a mild cytokine release. CRS mostly leads to problems with dosing, Biomunex CSO Simon Plyte said at PEGS Europe (5-7- November, Barcelona).
According to Plyte, cytotoxic MAIT cells preclinically circumvented T-cell-born safety issues, thus widening the therapeutic index of T cell therapies. While the proprietary, bispecific BiXAb antibody-mediated redirection of these MAIT cells to TAAs lead to the elimination of cancer cells with a potency identical to that of classical CD3epsilon T-cell engagers, MAIT engagers triggered neither cytokine release syndrome nor regulatory T-cell activation, because they are activated through binding to the iT cell receptor. According to data shown by Plyte in Barcelona, tumour-resident MAIT cells are able to eliminate autologous tumour cells in a MAIT-engager mediated manner, and can infiltrate and kill tumour cells in patient-derived 3D models of cancer.
Developed under the use of Biomunex’s proprietary BiXAb platform, BMX-502 selectively engages MAIT cells to GPC3-positive tumours . “As we continue to grow Ipsen’s pipeline using a science-first approach to partnering across the ecosystem, we believe BMX-502 is a strong addition with first-in-class potential in solid tumors,” said Mary Jane Hinrichs, SVP and Head of Early Development at Ipsen. “This new MAIT-engager program will complement our existing TCE portfolio as we harness this next generation of T cell engagers to overcome treatment challenges, including dose-limiting toxicity, to bring transformational new medicines to people living with solid tumors around the world.” Dr. Pierre-Emmanuel Gerard, founder, President and CEO of Biomunex, added that the exclusive global licence agreement is the first validation of Biomunex’s MAIT-engager approach.


 Ipsen SA
Ipsen SA