French Brenus AS closes US$25m financing
French Brenus Pharma SA has raised €22.2m (US$25m) to accelerate clinical trials of two precision cancer vaccines derived from tumour cell banks representing more than 200 tumour antigens.
The Series A round was led by French investors from the Auvergne-Rhône-Alpes (AURA) region, including UI Investissement/Kreaxi, Crédit Agricole Centre-France and Crédit Agricole Centre-Est, as well as Belgian funds Noshaq and Investsud. According to the Lyon-based company, the proceeds will be used to fund a multicentre Phase I/IIA trial of Brenus’ lead candidate, STC-1010, in French, Belgian and US patients with metastatic colorectal cancer that has become resistant to immunotherapy, and to accelerate the development of STC-1020 in “another solid tumour indication”.
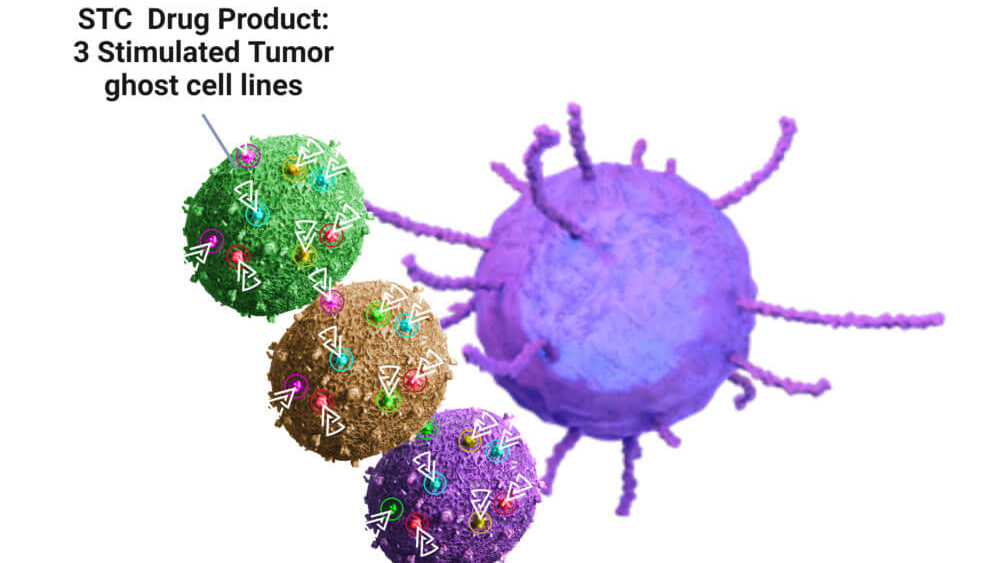
Both programmes are based on the patented Stimulated Tumour (ghost) Cell (STC) platform, which enables dendritic cells in patients to detect and anticipate escape and resistance mechanisms in patients’ cold tumours. The Stimulated Tumour Cells technology is the first platform to generate cancer vaccines based on cells that are stimulated to reproduce antigenic signatures of recurrent tumours and haptenised to train the immune system against resistant tumours.
Specificalls, the company uses non-proliferative haptenised allogeneic tumour ghost cells as a vaccine to evade therapy resistance and escape mechanisms of tumours. The tumour ghost cells carry exactly the same haptenised stress protein pattern on their surface that the individual patient’s tumour produces when exposed to chemotherapy, radio- or cancer immunotherapy, metabolic and oxidative stress. The method invented by Brenus Pharma’s CSO and co-founder Benoît Pinteur identifies this protein pattern by successively exposing patient cells to different types of stress and converting the resulting heat shock protein patterns into a form recognisable by the immune system, which is mapped onto the tumour ghost cells.
“After testing the STC product in colorectal cancer models in syngeneic immunocompetent mice (in vivo), where results showed improved overall survival and enhanced immune response with STC-1010, we have been testing new models (ex vivo, in ovo) to improve extrapolation to humans, validate the biological mechanism of action of STC-1010 and replicate the excellent results in human colorectal cancer models,” said Paul Bravetti, CEO of Brenus Pharma. 2All our efforts are now focussed on regulatory approvals and batch production for the clinical trial in 2024.”




 Freepik.com
Freepik.com