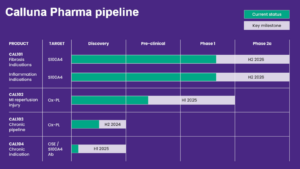
Cough medicine reverses fibrosis in animal models
Screening a library of 712 market-approved drugs, German researchers have found that a cough medicine most efficiently reduced incurable lung fibrosis in mice and in vitro.
A cough suppressant has reduced collagen deposition in mouse lung fibrosis, according to a screening of 712 FDA-approved drugs in human lung fibroblasts. Muzamil Khan and colleagues discovered that the common cough suppressant dextromethorphan can reduce the deposition of fibrillar collagen, which triggers the excessive tissue “scarring” known as fibrosis that can’t be reversed by market-approved medicines.
Fibrosis can affect various organs, including the lungs, where it is known as interstitial lung disease. The researchers from European Molecular Biology Laboratory tested the effects of dextromethorphan in an ex vivo human lung slice model and a mouse model of lung fibrosis. They found that the drug reduced lung fibrosis, which can ultimately lead to organ failure, in both therapeutic and preventative treatment regimens in mice.
Their findings could open up new treatment avenues to explore in lung fibrosis, as there are no curative therapies and the drugs currently used are expensive and can have significant side effects. Khan et al. note that dextromethorphan blocks the secretion of collagens and collagen-like cargo secretion in the extracellular matrix (ECM) through its effects on membrane trafficking of these proteins.
The reduced extracellular fibrillar collagen upon DXM treatment was due to reversible trafficking inhibition of collagen type I (COL1) in the endoplasmic reticulum (ER) in TANGO1- and HSP47-positive structures. Mass spectrometric analysis showed that DXM promoted hyperhydroxylation of proline and lysine residues on various collagens (COL1, COL3, COL4, COL5, COL7, and COL12) and latent transforming growth factor–β–binding protein (LTBP1 and LTBP2) peptides, coinciding with their secretion block. Additionally, proteome profiling of DXM-treated cells showed increased thermal stability of prolyl-hydroxylases P3H2, P3H3, P3H4, P4HA1, and P4HA2, suggesting a change in their activity. Transcriptome analysis of profibrotic stimulated primary human lung fibroblasts and human ex vivo lung slices after DXM treatment showed activation of an antifibrotic program through regulation of multiple pathways, including the MMP-ADAMTS axis, WNT signaling, and fibroblast-to-myofibroblast differentiation.
The researchers suggest that dextromethorphan might be a useful tool in studying the regulation and composition of ECM, as well as “a potential therapy for treating pulmonary fibrosis or other ECM-dependent diseases in the future.”
To get a weekly overview of the most relevant biomedical news, subscribe to our free newsletter.



 Adobe stock_DMH
Adobe stock_DMH