Actithera: Radiopharma Funding Gathers Huge Momentum
The biotech company Actithera, with locations in Cambridge, USA, and Oslo, Norway, has successfully closed a major Series A round, raising US $75.5 million. Its isotope-agnostic platform — designed with a specific focus on the interaction between linker and target molecule — has attracted strong interest from a wide range of investors. However, the path to this achievement was not as straightforward as anticipated, CEO Andreas Goutopoulos told European Biotech News.
Actithera, a radiopharmaceutical biotechnology company translating principles of medicinal chemistry into the next generation of radioligand therapies (RLTs), today announced the successful closing of an oversubscribed Series A financing round totaling US $75.5m. The funding will support the clinical development of the company’s lead FAP-targeted (Fibroblast Activation Protein) candidate across multiple oncology indications, while further advancing Actithera’s proprietary discovery platform and expanding its preclinical pipeline.
Strategic Investment Consortium
The Series A was co-led by founding investor M Ventures – the corporate venture unit of German Merck KGaA – and new lead investors Hadean Ventures, Sofinnova Partners, and 4BIO Capital. The investor syndicate also includes Bioqube Ventures, Surveyor Capital, Innovestor, Investinor, and co-founding investor Arkin Bio Ventures II. This diverse and experienced group reflects a newly growing global confidence in the therapeutic potential of radioligand technologies, which will also be seen in just another headline producing financing activity in due time.
Asking different questions
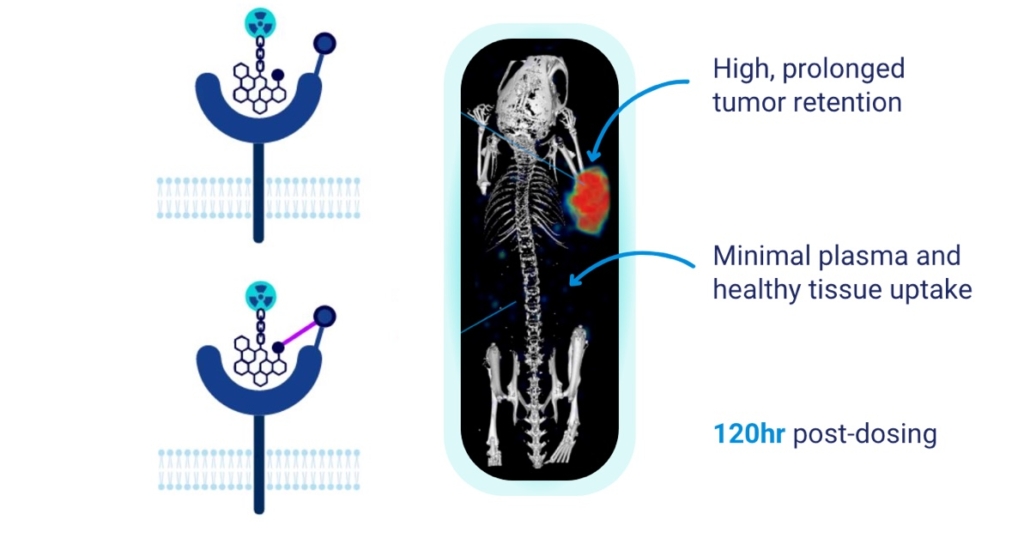
Under the scientific leadership of founder and medicinal chemistry expert Dr. Andreas Goutopoulos, Actithera has developed a first-in-class, tri-modular discovery platform designed to overcome critical limitations in radiopharmaceutical development. In a talk with European Biotech News, he stated, that his interest in the radiopharma space developed with the early success of Pluvicto and other therapies gaining approval. But as a chemist, he always wondered why only little attention was paid to the binding properties of the radiopharma molecule to the target itself. That´s why he focused on the topic ‘covalency’ and that´s why his platform integrates:
- Covalent targeting strategies: Pioneering first-in-class approaches to significantly extend tumor residence time while ensuring rapid systemic clearance.
- Optimized tumor pharmacokinetics: Enabling improved precision, safety, and efficacy of RLTs.
- Differentiated compound design: Enhancing tumor selectivity and residence through novel, chemistry-based mechanisms.
Goutopoulos´ platform has been validated through Actithera’s work on FAP — a high-value theranostic target notoriously difficult to address with long-retention molecules. The resulting FAP-targeted development candidate demonstrates best-in-class potential, combining high tumor specificity with a favorable pharmacokinetic profile ideal for durable radionuclide pairing. While just a few years ago it was relatively easy to secure funding for almost any radiopharma concept, the field has since cooled noticeably, Goutopoulos told EBM. Today, only mature or highly differentiated projects are managing to attract the necessary capital for further development. And that´s what Actithera was able to show in the investors consortium.
Long track record
Dr. Andreas Goutopoulos, Actithera’s CEO, brings over 25 years of experience in pharmaceutical and biotech R&D, including leadership of over a dozen development candidates. Prior to founding Actithera, he served as Head of Medicinal Chemistry at EMD Serono at the Boston site (USA) and was Entrepreneur-in-Residence at M Ventures. His approach at Actithera integrates covalent targeting, rational drug design, and an isotope-agnostic development philosophy to unlock the full potential of next-generation radiotherapeutics.
“We set out to bring structure-based and kinetics-driven thinking from small molecule drug design into the world of radiopharmaceuticals,” said Dr. Goutopoulos. “This oversubscribed Series A, backed by a truly global and experienced investor syndicate, is strong validation of our approach. We engineer our radioconjugates for extended retention within tumors, making them ideally suited for longer-lived radionuclides and ultimately delivering more convenient dosing schedules and enhanced efficacy and safety for patients”
Form the investor´s perspective, Karl Naegler, incoming board member and Partner at Sofinnova Partners, stated: “Actithera is applying Big Pharma discipline to an emerging field with enormous potential. Its radioligand therapies represent a meaningful shift in oncology, with the opportunity to redefine the therapeutic index. We’re excited to support that vision.”
Hakan Goker, current Chairman of Actithera and Managing Director at M Ventures, was the door opener and longtime supporter with funding the very first steps of a single-chemist start-up which Actithera was in the beginning. He said: “We are excited to see Actithera evolve from the one-person ideation we seeded with Andreas and Arkin to the transatlantic company it has become today. The innovative chemistry platform built and the first-in-class approach on FAP have the potential for a large impact in the RLT field and a significant benefit for patients. We welcome the new investor group and Board members to the company aligned with this bold vision of building the next generation of RLTs.”
Actithera will be headquartered in Oslo, Norway, with operations in Cambridge, Massachusetts, and is still hiring team members who will be situated in the laboratory. In Oslo, lab staff will work on broadening the discovery platform and strengthen the two other pillars of Actithera, which Andreas Goutopoulos didn´t disclose. But beyond covalency he may have found another major topic for better success and specificity of RLT, he told EBM. More news to come soon. For now, the money will be put into proceeding with preparations for clinical development which is expected to start in 2026 as a basket clinical trial, as many different cancer indications can be addressed with the FAB target in a very specific way. Radiopharma strikes back again tacking into account the next news of a huge financing round with Nuclidium AG from Switzerland in the very same week.


 Van Overeem Nuclear BV
Van Overeem Nuclear BV Nuclidium AG
Nuclidium AG