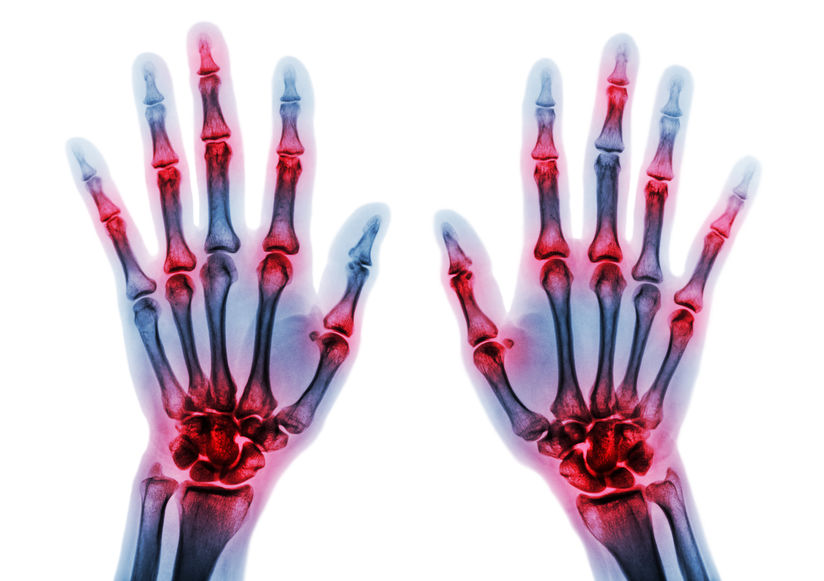
A novel strategy against rheumatoid arthritis
Researchers at AstraZeneca, Viela Bio, Gilead Sciences, Antidote Therapeutics Inc., and at SoseiHeptares report on a new small molecule compound to treat RA.
In a Phase 1b clinical trial of 42 patients with rheumatoid arthritis (RA), the CD40L-targeting compound VIB4920, developed at AZ/Medimmune spin-out Viela Bio, reined in autoimmune responses and alleviated symptoms. It proved safe and avoided the dangerous side effects such as thrombosis that were associated with previous antibody-based candidates targeting the CD40-CD40L axis, which is involved in autoimmunity.
VIB4920 is an anti-CD40L-Tn3 fusion protein that blocks human CD40L and inhibited the activation of B cells growing in culture without triggering platelets to aggregate into harmful clots. Furthermore, VIB4920 tamped down immune responses directed against the body and was well-tolerated in a Phase 1a study that enrolled 44 healthy volunteers. In the larger Phase 1b trial involving 42 RA patients and 15 controls, the treatment curtailed inflammatory biomarkers and reduced symptoms such as joint swelling after 12 weeks of treatment. Importantly, neither trial reported any instances of thrombosis or other severe adverse effects, indicating the molecule could potentially be developed into a newer and safer therapeutic for RA and other autoimmune diseases.
RA, affects 1.5 million US Americans alone.



 adobe.stock.com - ipopba
adobe.stock.com - ipopba BioDlink
BioDlink