High-Quality HIV Antigens for Cutting-Edge Solutions
ADVERTISEMENT
Neurodegenerative diseases like Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) suffer from a scarcity of effective treatments. Current therapies primarily provide symptomatic relief but do not address the underlying pathology, failing to slow or halt disease progression. This underscores the urgent need to identify new therapeutic targets relevant to the pathology of these conditions. Kinases represent highly tractable drug targets, with kinase inhibitors achieving significant success, particularly in oncology. However, their potential in neurodegenerative diseases remains largely unexplored, presenting a promising avenue for future research and therapeutic development.
Medix Biochemica expands its offering of critical in vitro diagnostics (IVD) raw materials to premium blockers and stabilizers by acquiring CANDOR Bioscience.
Lille hosted the 2024 editions of BioFIT, MedFIT, and MEDigIT, three internationally recognised events dedicated to innovation in Life Sciences, MedTech and Digital Health. These gatherings serve as catalysts for collaboration and progress, each focusing on a specific aspect of the healthcare innovation landscape.
AATec Medical was founded in 2022 and is located in the Innovation and Start-up Centre IZB in Planegg-Martinsried.
Ultra-low freezers are playing a key role in biomedical research as they are required to store very temperature-sensitive biologicals such as DNA, enzymes and more, but they have high energy demands. Recognizing these issues, B Medical Systems has introduced the U701V, a new ULT freezer that balances energy efficiency with exceptional performance.
The volume of samples and drug products requiring -80°C storage continues to increase, and shows no sign of slowing down. For many organisations, meeting these growing ULT storage requirements is challenging. The sheer volume of samples necessitates extensive storage capacity, which demands significant space, substantial energy use, and intensive management. Centralising and automating sample storage can provide a comprehensive solution.
Multiple sclerosis is currently an incurable, chronic autoimmune disease. While it is possible to effectively manage the feared relapses with existing therapies, novel approaches aim to provide important protection against long-term impairments.
Since 1995, the Eppendorf Award for Young European Investigators, endowed with €20,000, has honored outstanding achievements in the field of biomedical research in Europe once a year.
Biomanufacturers must balance the development of new therapeutic modalities and reduce manufacturing costs while maintaining environmental responsibility. Efforts are centred on improving process robustness and efficiency and creating more flexible manufacturing facilities that can accommodate diverse biopharmaceutical products.


 Sino Biological
Sino Biological
 Kerttu Penttilä Photography
Kerttu Penttilä Photography Léane Scemama/ BioFIT
Léane Scemama/ BioFIT AATec Medical GmbH
AATec Medical GmbH
 AZENTA
AZENTA Immunic/Nela Dorner
Immunic/Nela Dorner
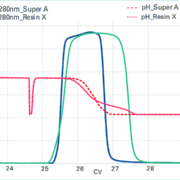 Tosoh Bioscience
Tosoh Bioscience