Abionyx Pharma SA has announced positive data from Phase IIa testing of iCER-001, a recombinant apoA-I, as a treatment for septic patients at high risk of developing Acute Kidney Injury (AKI)
ADVERTISEMENT
BioNTech SE will pay £562m in cash to acquire its former partner InstaDeep Ltd. to expand its capabilities in AI-based drug discovery.
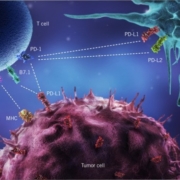
Roche AG’s US subsidiary Genentech Inc has withdrawn its checkpoint inhibitor atezolizumab from the market after disappointing results in bladder cancer.
Evosep APS has inked a $40m funding deal with Novo Holdings A/S that is aimed to advance standardised clinical LC-MS-based drug discovery and diagnostic multi-marker tests.
With the help of a €25m loan from the European Investment Bank, ExeVir BIO BV will advance clinical trials with its COVID-19 nanobody XVR012.
Rational antibody design specialist Hummingbird Bioscience has licenced a cancer target and the modular ADC technology of Dutch Synaffix BV.
Cipla EU Ltd is going to make an equity investment of €15m in Ethris GmbH, a specialist for respiratory delivery of therapeutic mRNAs.
Scientists from the University of Basel and the University Hospital Basel have found that two bacterial species may indicate multiple sclerosis-associated lymphopenia.
CEPI fights the betacoronaviruses by funding Ethris GmbH and DIOSynVax Ltd and their joint effort to advance their work on an mRNA vaccine.
Atara Biotherapeutics Inc. and Pierre Fabre SA announced the approval of Tabelecleucel, a therapy for a rare and potentially fatal malignancy.