Drug developer Confo Therapeutics and Eli Lilly & Company have inked a worldwide licensing agreement to co develop Confo’s neurophatic pain candidate CFTX-1554.
ADVERTISEMENT
The fourth fund of High-Tech Gründerfonds has reached a final fund volume of €493.8m
British anti-aging therapeutics developer 5 Alarm Bio Ltd has raised £500K in a seed financing for testing its lead compound FAB001 for healing chronic wounds.
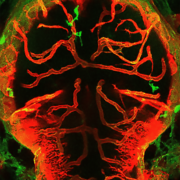
Interferon-gamma overexpression leads to hypertension-induced damage to the blood-brain barrier by reprogrammed macrophages.
French Abivax SA has raised €130m capital led by TCGX to accelerate Phase III testing of its ulcerative colitis candidate obefazimod extend its cashflow.
Myoglobin producer Paleo NV has closed a € 12 m Series A round led by DSM Venturing and Planet A Ventures.
CDMO Minaris Regenerative Medicine GmbH is to manufacture the leukocyte infusion therapy N-LIfT for LIFT BioSciences.
PVH Corp has joined fiber-to-fiber consortium that wants to make textile recycling sustainable by establishing standards for biorecycled textile fibre.
A UK Biobank study suggests that higher intake of free sugars is associated with a higher risk to develop cardiovascular disease, UK researchers report in BMC Medicine.
Fermatation expert Evonik SE has entered into an agreement with the German recombinant silk producer AMSilk to produce industrial quantities of innovative, sustainable silk proteins.






 © peterschreiber. media - stock.ado
© peterschreiber. media - stock.ado